Protein can release trapped histones in the cell
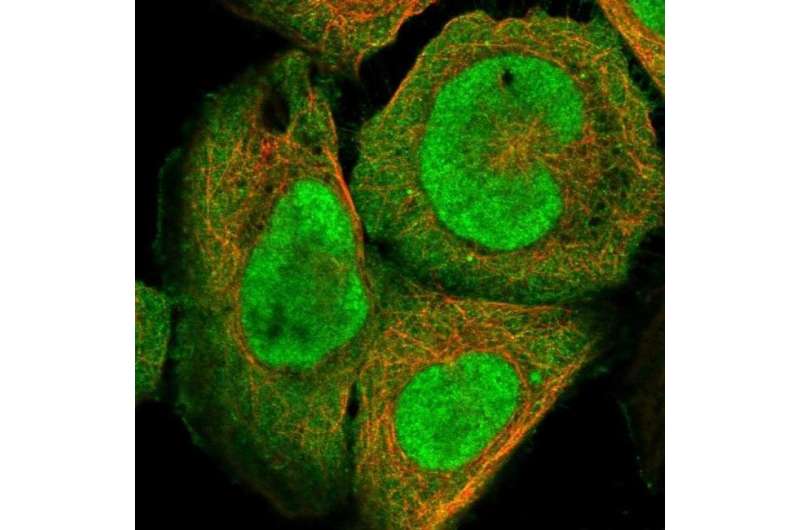
In the cell nucleus, histones play a crucial role packaging DNA into chromatin. Histones are however very sticky to both DNA and RNA, so to ensure they are transported to the cell nucleus after synthesis and bind to the right portion of DNA to organize the chromatin, they are guarded by complexes of histone chaperones.
Histone chaperones are proteins that bind to histones to help protect them from non-specific binding events until they reach their goal. This process fails sometimes and histones get stuck during their supply to chromatin without any purpose.
In a study published in Molecular Cell, researchers have shown that the protein DNAJC9 holds an important role in safeguarding histones and thereby chromatin.
Active fixer joins passive bystanders
"Until now, researchers have assumed that histone chaperones only act to passively shield histones. We have found out that DNAJC9 actively engages the cellular protein folding machinery—which means it actively recruits enzymes and molecular chaperones to redeploy histones that have been trapped," says assistant professor Colin Hammond, who has lead the study. Hammond is part of the Protein Memory Program in the Novo Nordisk Foundation Center for Protein Research at University of Copenhagen.
Once released from their trapped condition histones can re-engage histone chaperones like MCM2 and be assembled into nucleosomes to organize chromatin. When the protein DNAJC9 is mutated to lose its ability to recruit the protein folding machinery, the histones stay trapped and are thereby lost for proper chromatin deposition.
"This means that traditional histone chaperones cannot fully protect histone proteins from spurious interactions. Rather, the cell is dependent on the combined action of molecular chaperones and histone chaperones to safeguard these fundamentally important proteins during their dynamic lives," Colin Hammond underlines.
Strong collaboration necessary
The study is based on a strong international collaboration. Co-first author Hongyu Bao in Hongda Huang's lab and other partners from Southern University of Science and Technology in China, and Dinshaw Patel from Memorial Sloan Kettering Cancer Center, provided the structural discoveries of DNAJC9. The functional analysis was spear-headed by Colin Hammond in close collaboration with colleagues from the Proteomics Program at CPR, Ivo Hendriks and Michael Lund Nielsen, to dissect the function of the protein through in depth proteomic assays.
Relevant insights for cancer research
DNAJC9 is an essential protein in many cancer cell types and the levels of the protein correlate with the rates at which cancer cells proliferate. Chromatin in cancer cells may be more reliant on DNAJC9 compared to regular cells, and if this is the case DNAJC9 could be a target for the development of future cancer treatments.
"Although it's still early days, we hope this fundamental advance in our understanding of DNAJC9 biology helps to pinpoint a function essential for cancer cell viability with therapeutic potential," Colin Hammond says.
More information: Molecular Cell (2021). DOI: 10.1016/j.molcel.2021.03.041
Journal information: Molecular Cell
Provided by University of Copenhagen