Turing structures in a manmade interface
In 1952, Alan Turing, the father of computer science and artificial intelligence, proposed that certain repetitive natural patterns may be produced by the interaction of two specific substances through the reaction-diffusion process. In this system, an activator promotes the reaction and an inhibitor inhibits the reaction. When the two meet, the reaction diffuses. When the difference in diffusion coefficient between the two reaches a certain level, the high diffusion ratio between them will cause system imbalance and induce the formation of periodic complex patterns.
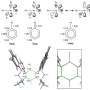
'Turing structures' exist widely in nature, such as the body patterns of zebras, the phyllotaxis of sunflowers, the follicle spacing of mouse hairs and others. However, it is difficult to construct a Turing structure in a manmade chemical system since the difference in diffusion coefficients of substances is small.
Recently, the research group of Prof. Gao Minrui from the University of Science and Technology of China created the Turing structure on inorganic transition metal chalcogenides with the reaction-diffusion process for the first time. Results were published in Angewandte Chemie International Editionand selected as a Hot Paper and Back Cover.
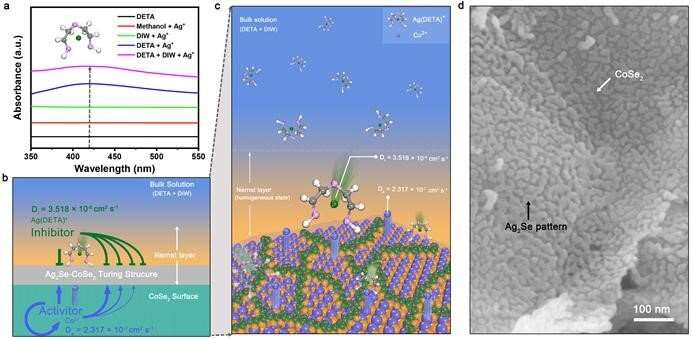
In the binary solution of diethylenetriamine (DETA) and water, Ag+ will react with DETA to form Ag(DETA)+. At the same time, Co2+ overflows from the surface of a cobalt diselenide (CoSe2) nanobelt. Ag(DETA)+ is the inhibitor and Co2+ is the activator in this system. When the rapidly diffused Ag (DETA)+ reaches the Nernst layer on the CoSe2 surface, it interacts with the activator Co2+ diffused on the CoSe2 surface, and finally forms a complex and beautiful Ag2Se Turing pattern on the CoSe2 surface.
The study found that this multi-interface Turing structure material, Ag2Se-CoSe2, was an efficient oxygen evolution reaction (OER) electrocatalyst. The OER activity of Ag2Se-CoSe2 was linearly related to the interface length of the Turing structure. The rich interface structure and the optimized OER intermediate adsorption energy at the interface structure conspired to bring about its high activity.
This study uses the reaction-diffusion theory to construct complex Turing structures on inorganic nanostructured materials for the first time, and provides new ideas for the design of cheap catalysts with higher performance.This research employed the reaction-diffusion theory to build a complex Turing structure on inorganic nanostructured materials for the first time, and provided a new path for designing cheaper catalysts with higher performance.
More information: Xiao‐Long Zhang et al, An Efficient Turing‐Type Ag2Se‐CoSe2 Multi‐Interfacial Oxygen‐Evolving Electrocatalyst**, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202017016
Journal information: Angewandte Chemie International Edition
Provided by University of Science and Technology of China