March 19, 2021 report
Using a nanoscale tandem catalyst to get more propylene out of propane during dehydrogenation
A team of researchers at Northwestern University has developed a nanoscale tandem catalyst to get more propylene out of propane during dehydrogenation. In their paper published in the journal Science, the group describes their method and the improvements they found in its use. Chunlei Pei and Jinlong Gong with Tianjin University have published a Perspectives piece in the same journal issue outlining the benefits of tandem catalysis and the work done by the team in Illinois.
Businesses that use chemistry to create products have found over the years that reducing the number of steps required to make their products quite often results in money savings. This has led chemists to investigate the possibility of integrating multiple steps into single reactions—such tandem reactions involve sequential actions to bring about desired results. In this new effort, the researchers have developed a tandem reaction to reduce the number of steps required to produce propylene during dehydrogenation of propane, and in so doing, have increased yield. Propylene is a gaseous hydrocarbon that is used to make several types of polymers.
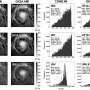
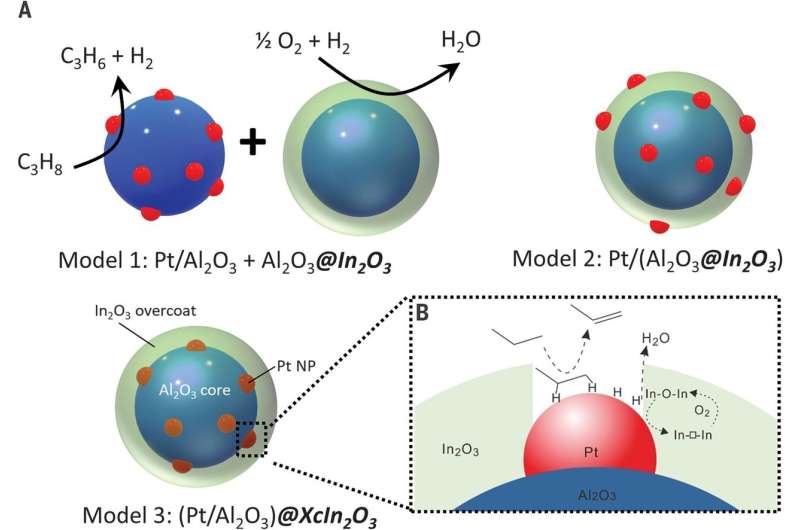
The work involved developing a nanoscale catalyst that used an overcoat to allow for increased surface oxidation of hydrogen atoms—the overcoats were approximately 2 nanometers thick. To create the overcoats, the researchers used atomic layer deposition as a means of growing indium oxide over Pt/Al2O3—a known propane dehydrogenation catalyst. This caused domain coupling via surface hydrogen atom transfer—and that resulted in propane dehydrogenating to propylene by platinum and increased hydrogen combustion from the indium oxide. The researchers note that oxidation was improved due to the pores that developed in the overcoating allowing greater exposure of the platinum nanoparticles—hydrogen atoms on the surface were better oxidized at the platinum-indium oxide interface. The researchers found that use of their tandem catalyst resulted in 75% propylene selectivity and propane conversion of 40%, boosting yields by approximately 30%. Pei and Gong suggest the results should inspire further work both in industry and academia because it likely could be used in many other applications.
More information: Huan Yan et al. Tandem In2O3-Pt/Al2O3 catalyst for coupling of propane dehydrogenation to selective H2 combustion, Science (2021). DOI: 10.1126/science.abd4441
Chunlei Pei et al. Tandem catalysis at nanoscale, Science (2021). DOI: 10.1126/science.abh0424
Journal information: Science
© 2021 Science X Network