Small, single-stranded genetic material may account for zoonotic COVID transmission
A study led by researchers at University of Westminster shows that small single stranded genetic material may play a role in how COVID-19 (SARS-CoV-2) passes from animals to humans and why some animal carriers of the virus may show no symptoms while it can be deadly in humans.
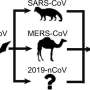
The findings, published in the journal Viruses in collaboration with Gebze Technical University and University of Bedfordshire, indicate that in particular pangolins and bats, but also cows and pigs, are the most likely carriers of the virus capable of passing it on to a human, known as zoonotic carriers.
The study also provides new insights into how the immune systems of various animal species (bat, pangolin, cow, chicken, mink, rat) may tolerate the virus differently to humans, and also points at specific changes occurring in such genetic material in the pangolin, which may explain severe symptoms in humans.
The SARS-CoV-2 virus has been found to cause a range of, including severe, symptoms in humans, while many animals which are suspected to be carriers of the virus remain unaffected and act as a reservoir for the virus. The reason for this difference still remains unclear.
The researchers assessed small genetic material known as microRNAs, which can help to regulate the immune system. They assessed seven key microRNAs, which they had previously identified to be shared between the SARS-CoV-2 genome and the human genome. They then looked at the same microRNAs in some of the main wild and domestic zoonotic species reported for human viruses.
These seven microRNAs regulate genes that play important roles in viral-host interactions and other relevant cellular and immunological processes.
They revealed differences in these microRNA sequences between the different suspected zoonotic carriers compared with humans, indicating possible roles for these microRNAs in the coevolution of the virus and the host. The results also highlight pangolin, bat, cow, and pig as likely zoonotic carriers, and point to specific changes in the pangolin, which may affect disease severity of COVID-19 in humans.
This is the first study to assess microRNAs in relation to possible animals that can be carriers for the virus and is identifying this as a new mechanism that may contribute to disease severity, and tolerance in other species.
The findings may contribute to the current understanding of some of the detrimental effects observed by human host immune responses when encountering new zoonotic pathogens and pave the way for further investigations into the roles of microRNAs in zoonosis.
Dr. Sigrun Lange said: "Comparing immune systems and their regulation between species is of great importance as some viruses can jump from wild to domestic animals. This facilitates transmission of dangerous new viruses from animal to human hosts where responses to these emerging diseases can be detrimental, as we have seen in COVID-19."
Dr. Uysal-Onganer said: "The role of microRNAs in the regulation of host-pathogen interactions is a vastly underexplored topic with a huge knowledge gap in relation to zoonosis. These small cast-off parts of our genetic material can cause changes in how many processes in the body are regulated, including in viral infection. They can regulate host immune response, which leads to different clinical outcomes.
"Our findings contribute to current understanding of some of the detrimental effects observed by human host immune responses when encountering a new zoonotic pathogen like SARS-CoV-2, while animals carrying the virus may be less or unaffected. To target microRNAs in emerging infectious diseases may be a promising strategy for novel therapeutic intervention."
More information: MicroRNAs for Virus Pathogenicity and Host Responses, Identified in SARS-CoV-2 Genomes, May Play Roles in Viral-Host Co-Evolution in Putative Zoonotic Host Species. Viruses, doi.org/10.3390/v13010117
Provided by University of Westminster