Manipulating chain-walking in olefins using earth-abundant iron-based catalysts
NUS chemists have discovered a way of controlling the position of carbon-carbon double bonds (C=C) in olefin isomerisation using sustainable iron-based catalysis for potential applications in organic synthesis.
The catalytic isomerisation of C=C bonds is an indispensable chemical transformation used to deliver higher-value analogs. It has important uses in the chemical industry. Although a vast number of metal-catalyzed C=C bond migration protocols have been developed, enduring shortcomings remain unresolved. The capability to reposition the C=C bonds within olefins, known as alkene transposition, is an efficient approach to obtain structurally isomeric forms of the same molecule from a single substrate. However, this continues to be a formidable challenge. Separately, the conversion of regioisomeric alkene mixtures which contain many isomers of the same compound to a single product having only a single isomer, is yet to be achieved. This conversion is particularly useful for streamlining chemical synthesis.
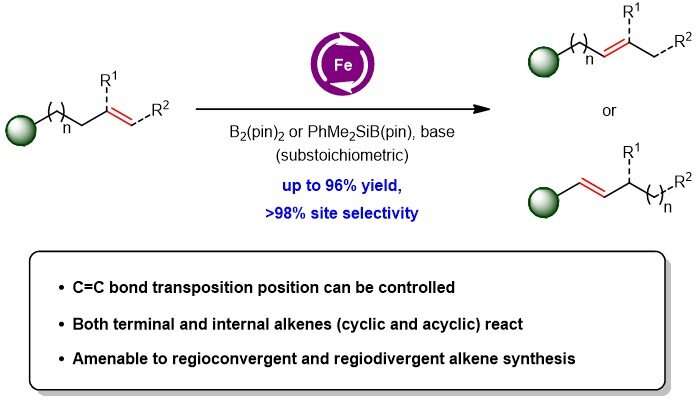
A research team led by Prof Koh Ming Joo, from the Department of Chemistry, NUS has devised a new olefin isomerisation technique in which catalytic amounts of an appropriate earth-abundant iron-based complex, a base and a boryl compound are employed to promote efficient and controllable alkene transposition (see Figure 1). This method can be used for both terminal and internal alkene substrates. Mechanistic studies by the team revealed that these chemical transformations are likely to proceed through a catalytic iron-hydride intermediate that induces C=C bond migration.
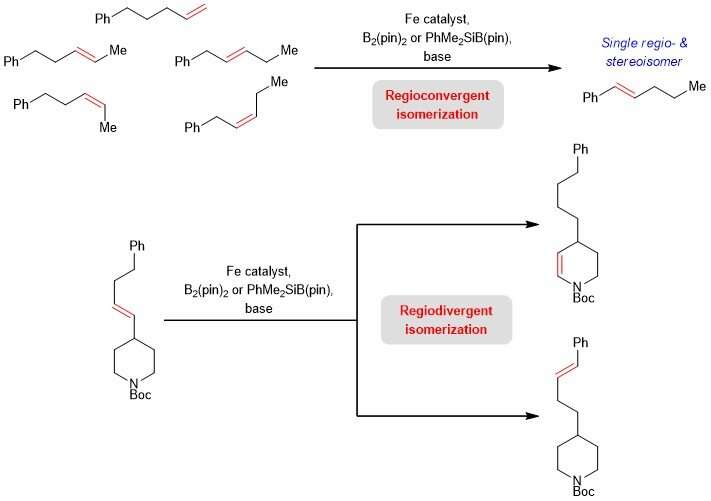
Prof Koh said, "The new catalytic regime provides a way to obtain structurally different products from a single substrate. Our team also found that iron-based catalysis can also be used to transform isomeric olefin mixtures, which are commonly found in petroleum-derived feedstock to a single alkene product for use by the petrochemical industry. This scientific advance not only highlights a new approach for alkene chain-walking. It also illustrates the viability of controlling where the C=C bond migrates even in substrates bearing at least two possible sites that may direct the olefin isomerisation. This has significant implications to facilitate the synthesis of unsaturated moieties embedded within biologically active compounds."
The research team plans to combine this new methodology with olefin addition reactions to develop novel functionalisation transformations.
More information: Xiaolong Yu et al. Iron-Catalyzed Tunable and Site-Selective Olefin Transposition, Journal of the American Chemical Society (2020). DOI: 10.1021/jacs.0c08631
Journal information: Journal of the American Chemical Society
Provided by National University of Singapore