Artificial photosynthesis can convert useless carbon dioxide into formic acid used in industry

With energy from the sun, a special enzyme can convert CO2 molecules into formic acid. This can both remove CO2 and provide us with something more useful.
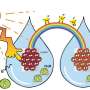
The photosynthesis of plants is one of nature's many wonders. Using the energy of the sunlight, carbon dioxide (CO2) and water are converted into sugar and other carbohydrates, as well as oxygen.
This is done through a series of chemical processes. By mimicing parts or all of the photosynthesis, may we be able to emit less CO2 or capture some of what is floating around in the air?
All over the world, scientists are inspired by the photosynthesis. One of them is chemist Kaiqi Xu at the University of Oslo.
"We want to use artificial photosynthesis because natural photosynthesis is not always very effective," Xu says.
He does not say this to undermine nature's own chemical laboratory, but there is no doubt that there is room for improvement. For example, the plants utilize only 1-2 percent of the sunlight. A silicon solar cell utilizes between 15 and 24 percent.
"Natural photosynthesis can produce sugars from CO2and water. We want to produce something more useful," Xu tells Titan.uio.no.
His doctoral degree is a small step on the road to what can be an opportunity to control CO2levels.
Enzyme from virus or bacteria
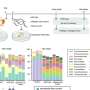
Xu has investigated an enzyme that can convert CO2 into formic acid, a substance used in several forms of industry.
Enzymes are a type of protein that act as catalysts in biological processes, both in your body, in plants and everywhere else. These have specialized in driving very specific reactions.
There are countless different enzymes. Xu calls his enzyme an oxygen-tolerant formate dehydrogenase, and it belongs to a group called FDH enzymes.
"The enzyme we use is produced by bacteria or viruses, but I also think some scientists use FDH enzymes directly from plants," Xu says.
Under the right circumstances, Xu's FDH enzyme can grab the CO2 molecule and convert it into formic acid. But for this it needs energy.
The same thing happens in a solar cell
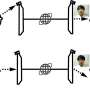
He obtains the energy from a nanotube made of tantalum nitride, Ta3N5, where each molecule consists of three atoms of the element tantalum and five nitrogen atoms.
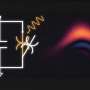
"Tantalum nitride is a semiconductor with unique properties. It can absorb sunlight and convert it into energy that can be directly utilized by us," Xu explains.
When the sunlight hits tantalum nitride, an exactly appropriate amount of energy is emitted. It's the same thing that happens in a solar cell. An electron jumps out, but where a solar cell wants throws the electron into a circuit, Xu wants it to drive the chemical reactions in the FDH enzyme.
"The enzyme can capture electrons generated from the tantalum nitride and then carry out the reaction," Xu says.
It is no coincidence that he uses tantalum nitride in his research.
"Tantalum nitride meets many of the requirements for performing photosynthesis," Xu says.
Partly because it has a band gap of 2.1 electron volts. Band gap is the energy needed to get an electron out of its ground state. This energy of 2.1 electron volts can power the overall photosynthetic process, including the energy needed by the enzyme to to its job.
"Then this enzyme can convert CO2 to formic acid, a composition that is much more valuable," Xu says.
In addition, we can get rid of some CO2, of course, which is no disadvantage in terms of climate change.
Can capture CO2
Xu makes very small tubes with tantalum nitride. So small that they are at nano level. Nano means one billionth.
"We make nanotubes of tantalum nitride because tubes have a very large surface area and thus can absorb more sunlight."
Perhaps the technology in the future can contribute to CO2 capture.
"If we can make it a thin layer, we can put it on roofs and walls that will then help capture CO2," Xu says.
"A lot we don't know"
But there is a lot of research needed before we get there. Xu's FDH enzyme still has many secrets.
"Now we know a little about this enzyme, but there's still a lot we don't know," he says.
"If we can get even better insight into the enzyme and if we can mimic it, we can do this on a larger scale. Then it can definitely help to control the CO2 level."
"If we can make it even more efficient, it can surpass the function of green plants," Xu says.
Provided by University of Oslo