Technique to make functional materials based on polymers of metal clusters
Researchers at the universities of Jyvaskyla (Finland) and Xiamen (China) have discovered a novel way to make functional macroscopic crystalline materials out of nanometer-size 34-atom silver-gold intermetallic clusters. The cluster material has a highly anisotropic electrical conductivity, being a semiconductor in one direction and an electrical insulator in other directions. Synthesis of the material and its electrical properties were investigated in Xiamen and the theoretical characterization of the material was carried out in Jyvaskyla. The research was published online in Nature Communications on May 6, 2020.
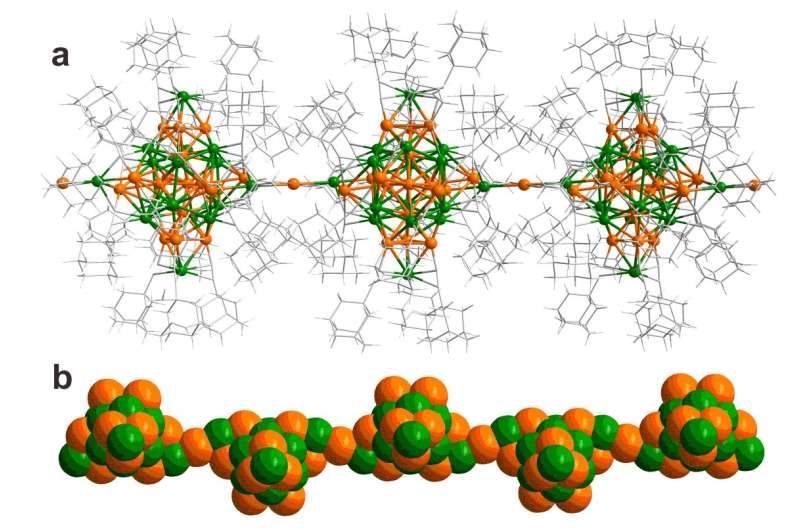
The metal clusters were synthesized by means of wet chemistry, adding gold and silver salts and ethynyladamantane molecules in a mixture of methanol and either chloroform or dichloromethane. All syntheses produced the same 34-atom silver-gold clusters with an identical atomic structure, but surprisingly, the use of dichloromethane/methanol solvent initiated a polymerization reaction after cluster formation in solution and growth of human-hair-thick single crystals consisting of aligned polymeric chains of the clusters.
The crystals behaved as a semiconducting material in the direction of the polymer and as an electrical insulator in the cross directions. This behavior arises from metal-metal atomic bonding in the polymer direction while in the cross directions the metal clusters are isolated from each other by a layer of the ethynyladamantane.
Theoretical modeling of the cluster material by computer-intensive simulations using the density functional theory predicted that the material has an energy gap of 1.3 eV for electronic excitations. This was confirmed by measurements of optical absorption and electrical conductivity in a layout where single crystals we mounted as part of a field-effect transistor, which showed a p-type semiconductor property of the material. Electrical conductivity along the polymer direction was about 1800-fold as compared to the cross directions.
"We were quite surprised by the observation that the polymer formation can be controlled by simple means of changing the solvent molecules. We discovered this probably by good luck, but we hope that this result can be applied in future to design hierarchical nanostructured materials with desired functionality," says Professor Nanfeng Zheng from Xiamen University, who led the experimental work.
"This work shows an interesting example on how macroscopic material properties can be designed in the bottom-up synthesis of nanomaterials. Theoretical modeling of this material was quite challenging due to a large-scale model we had to build to account for the correct periodicity of the polymer crystal. To this end, we benefited very much of having access to some of the largest supercomputers in Europe," says Academy Professor Hannu Hakkinen from the University of Jyvaskyla, who led the theoretical work.
More information: Peng Yuan et al, Solvent-mediated assembly of atom-precise gold–silver nanoclusters to semiconducting one-dimensional materials, Nature Communications (2020). DOI: 10.1038/s41467-020-16062-6
Journal information: Nature Communications
Provided by University of Jyväskylä