May 13, 2020 feature
A new electrolyte design that could enhance the performance of Li-ion batteries
Most existing lithium-ion batteries (LIBs) integrate graphite anodes, which have a capacity of approximately 350 milliamp hours (mAh) per gram. The capacity of silicon anodes is almost 10 times higher than that of their graphite counterparts (around 2,800 mAh per gram), and could thus theoretically enable the development of more compact and lighter lithium-based batteries.
Despite their higher capacity, silicon anodes have so far been unable to compete with graphite anodes, as silicon expands and contracts during battery operation, so the anodes' outer protective layer can easily crack while a battery is operating. In a recent paper published in Nature Energy, a team of researchers at the University of Maryland College Park and Army Research Laboratory has reported a new electrolyte design that could overcome the limitations of existing silicon anodes.
"Silicon anodes and their formed solid electrolyte interphase (SEI) protecting layers are easier to pulverize during battery operation, because the SEI strongly bonds to Si, so both experience a large volume of changes," Ji Chen, one of the leading researchers who carried out the study, told Phys.org.
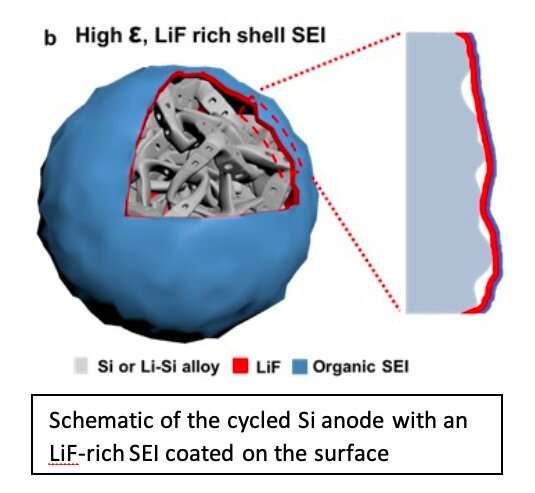
The SEI is a protective layer that forms naturally when anode particles are in direct contact with an electrolyte. This layer serves as a barrier that prevents further reactions from occurring inside the battery, separating the anode from the electrolyte.
"If this protective layer becomes damaged during Si anode particle expansion or contraction, the newly exposed anode particles react continuously with the electrolyte until it runs out during battery cycling," Oleg Borodin, a senior chemist involved in the study at Army Research Laboratory, told Phys.org.
For more than a decade, research groups worldwide have tried to overcome the issues preventing the use of silicon anodes in LIBs, primarily by designing flexible and organic SEIs that expand with the anodes. Most of the solutions they developed, however, have proved to be either entirely ineffective or mildly effective, thus only partly preventing SEI damage.
"For a long time, the LIB research community has been trying to devise techniques to make high-capacity anodes like Si work," said Chunsheng Wang, a professor at the Department of Chemical & Biomolecular Engineering and Department of Chemistry & Biochemistry of University of Maryland (UMD), who is also the UMD Director of the Center for Research in Extreme batteries. "These researchers were mostly working on the Si material level by introducing expensive nanofabrication processes. We tried to tackle this problem differently by designing the electrolyte and corresponding SEI for high-capacity anodes."
Chen, Borodin, Wang and their colleagues designed an electrolyte that could enhance the performance of microsized silicon anodes in LIBs, preventing damage to their outer protective barrier. Compared to previously proposed solutions, their approach substantially minimizes electrolyte degradation, thus allowing much longer battery cycling before it loses its capacity.
The ultimate goal of the researchers' study was to identify a universal, drop-in solution that could facilitate the development of high-capacity anodes for Li-based batteries. To achieve this, they designed electrolytes using LiPF6, a state-of-the-art salt, and a mixture of ether solvents, forming a very robust LiF-rich SEI protective layer.
"The special solvation structure (interaction between the salt and the solvent) and the wide gap between the reduction tendency of the salt and solvent promotes the formation of a unique LiF-rich SEI on Si that is super-helpful for cycling the high-capacity Si anodes," Oleg explained. "The electrolyte we designed provides a drop-in solution for the current LIB technology without requiring expensive processing, while maintaining a high cycling stability and Coulombic efficiency (CE) that is unprecedented."
The recent study by Chen, Borodin, Wang and their colleagues proves that achieving good cycling and high CE in LIBs containing silicon anodes is, in fact, possible, and that it can be achieved simply by replacing the electrolyte inside a battery, which was previously considered impractical or entirely unfeasible. The principle behind their electrolyte design could also theoretically be applied to all high capacity alloy anodes. In the future, this design could enable the creation of better-performing lithium-based batteries that contain anodes based on materials other than graphite.
"Our findings point out a new direction for electrolyte design and could give research teams worldwide confidence in the application of high-capacity anode materials in LIBs," Wang said. "Our next steps will be to improve the voltage window of the electrolyte and try to license the technology to battery manufacturers."
More information: Ji Chen et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries, Nature Energy (2020). DOI: 10.1038/s41560-020-0601-1
Journal information: Nature Energy
© 2020 Science X Network