April 22, 2020 feature
The surface stress of biomedical silicones is a stimulant of cellular response
![Silicone liquids and soft gels stimulate rigidity signaling responses. (A) Top row: Pictures of fourth-generation and fifth-generation breast implants, liquid silicone oil, and soft or rigid polyacrylamide (PA) and silicone gels. Lower rows: Representative phase-contrast images, cell masks, and Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) staining of MCF10A MECs on the indicated substrate with adsorbed fibronectin. Scale bars, 30 μm. Experiments were conducted in triplicate. PA soft, 0.12 kPa; PA stiff, 20 kPa; silicone soft, 0.1 kPa; silicone stiff, 21 kPa; liquid silicone, negligible elasticity. Photo credit: Zhu Cheng and Matthew Paszek, Cornell University. (B) Top: Representative immunoblot of total and phospho-FAK in MECs on the indicated substrates. Bottom: Quantification of the ratio of phospho-FAK to total FAK from immunoblot shown in the top panel. Error bars show SEMs. **P < 0.005 and ns, not significant [one-way analysis of variance (ANOVA) with Tukey’s post hoc test]; n > 4. (C) Representative immunoblot of total and phospho-FAK in MECs on the indicated substrates (top) and quantification of immunoblot signal (bottom). TCPS, tissue culture polystyrene. Error bars show SEMs. ns, not significant. Wilcoxon rank scores test was used; n > 4. (D) Quantification of the ratio of nuclear YAP signal to cytoplasmic YAP signal. ***P < 0.001, one-way ANOVA; n > 35 per condition. Experiments were conducted in triplicate. Horizontal lines are medians. Boxes show the interquartile range (IQR). Whiskers extend to minimum and maximum values. Credit: Science Advances, doi: 10.1126/sciadv.aay0076 The surface stress of biomedical silicones is a stimulant of cellular response](https://scx1.b-cdn.net/csz/news/800a/2020/thesurfacest.jpg)
Silicones are commonly used in the field of medicine to lubricate syringes, encapsulate medical devices, and develop surgical implants. Although the material is generally viewed as relatively inert to cells, they can trigger a variety of inflammatory responses and other deleterious effects—but the mechanisms underlying their bioactivity remain to be determined. In a new report, Zhu Cheng, and a research team in chemical and biomedical engineering at Cornell and Stanford University, U.S., detailed that silicone liquids and gels have high surface stresses to strongly resist deformation at cellular strengths. For instance, biomedical silicones used for syringe lubricants from FDA-approved breast implants can readily adsorb matrix proteins to activate canonical rigidity sensing pathways through their surface stresses. Using 3-D cell culture models, the bioengineers showed how liquid silicone droplets supported robust cellular adhesion to form multinucleated, monocyte-derived cell masses recapitulating characteristics of granuloma formation similar to those observed during a foreign body response. The findings are now on Science Advances and imply surface stress as a cellular stimulant, which should be considered in applications of silicones for future biomedical purposes.
The broad medical applications of silicones are credited to their ease of fabrication, mechanical properties that can be controlled and long usage history. In the United States, silicone gels are a popular implant material for cosmetic and reconstructive procedures. Although the material is regarded to be biocompatible and safe, multiple disease types including breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) are linked to the implant material. Patients also commonly undergo capsular contraction as a local response, which results in an excessive fibrotic foreign body reaction.
While biomedical silicones are typically assumed to have minimal capacity to stimulate mechanotransduction, all materials maintain a characteristic surface energy defined as the energy penalty per unit area. In a liquid, this gives rise to surface tension at the air-water interface, allowing insects such as fishing spiders to walk on water. In solids, surface energy can give rise to stresses that similarly resist deformation. In this work, Cheng et al. considered if the surface stresses of silicones could activate rigidity pathways to control downstream cellular responses. For this, the team considered biomedical silicones, liquid silicone oils and model silicone gel systems of varying bulk rigidity.
The team first tested the response of cells to soft silicone gels using fourth- and fifth-generation implantable devices approved from the U.S. Food and Drug Administration (FDA) for breast reconstruction and augmentation. They extracted the silicone fillings by puncturing a site on the outer shell of each implant and spread the extracts on the bottom of cell culture dishes. The extracts included a viscous silicone gel based on fourth-generation devices and a "gummy bear" silicone with fifth-generation filling. The scientists observed the extracellular matrix protein fibronectin to readily adsorb to the gel surface without surface treatments or coupling reagents. Mammary epithelial cells (MECs) cultured on the soft gels were also well spread—this outcome was highly unexpected since previous research had indicated suppression of cell spreading on compliant substrates due to insufficient stimulation of the rigidity sensing pathways.
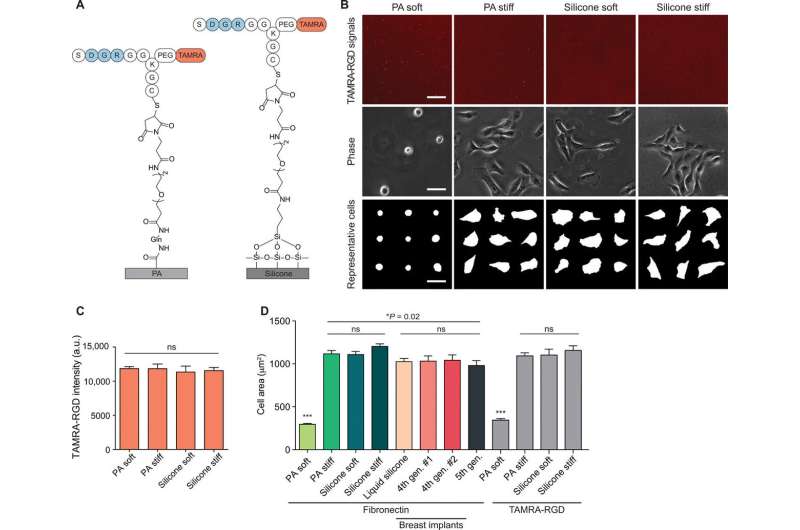
Cheng et al. used a model silicone gel system of controlled elasticity in the experiments. Fibronectin readily adsorbed on the silicone gels for cell spreading and stimulated the activation of mechanical cell signaling pathways such as phosphorylated focal adhesion kinase (FAKpY397). The scientists tested if rigidity signaling by silicones was functionally linked to transcriptional regulation (i.e. at the RNA level) and noted that cells on soft silicone gels exhibited nuclear Yes-associated protein (YAP) to turn on gene expression after activating rigidity sensing pathways. When the team blocked FAK-based rigidity signaling with pharmaceutical inhibition to validate their role—they noted stunted YAP translocation to the nucleus. Cheng et al. then engineered silicone substrates with adhesive peptides to test if ligand presentation stimulated a rigidity response and noted similar cell growth on peptide-conjugated substrates compared to fibronectin adsorbed surfaces.
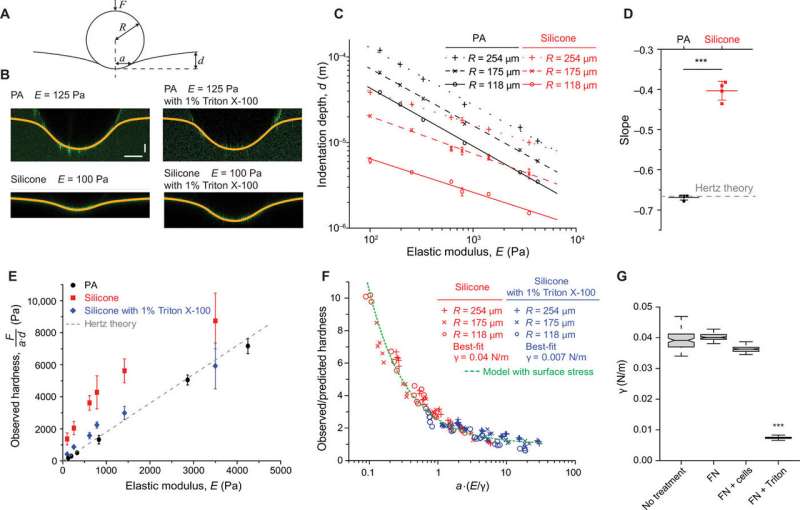
To understand the mechanisms underlying cell adhesion on silicone materials, the team examined surface stresses arising due to the surface energy at the material interfaces. They used contact angle measurements with water droplets during this experiment and noted higher surface energy for functionalized silicone substrates. To investigate the surface stresses, they used confocal fluorescence microscopy and measured surface indentation with small, spherical steel balls and noted resistance to surface deformation. When they repeated experiments after long-term cell culture the surface tension of the silicone substrates did not alter. Using multiple tests, the scientists indicated solid surface stress to be the primary stimulatory cue for liquid silicones and highly compliant gels.
During functional tests, Cheng et al. investigated the interactions between peripheral blood mononuclear cells (PBMCs) and their interaction with a common syringe lubricant liquid silicone oil – polydimethylsiloxane (PDMS). When PBMCs assembled at the silicone oil and collagen gel interface, they fused to form giant multinucleated cells, resulting in characteristic foreign body reactions that are typically observed with incompatible, implanted biomaterials.
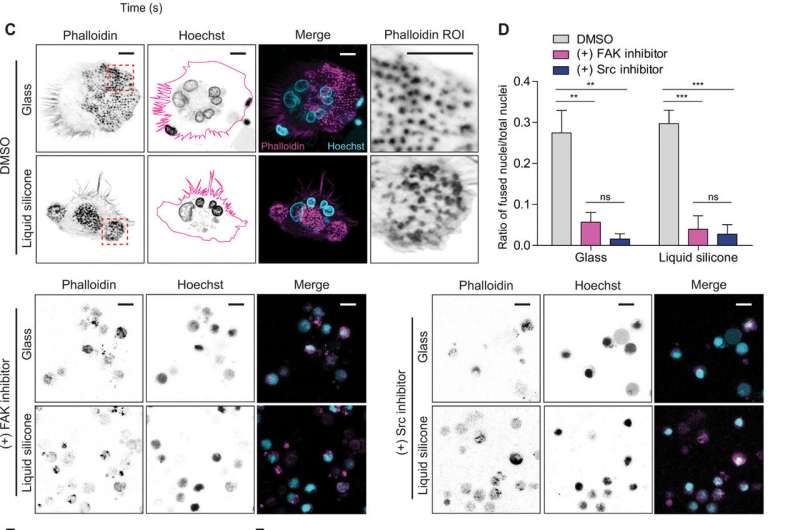
In this way, Zhu Cheng, and colleagues revealed material surface stress as a potent mechano-stimulant to control diverse biological behaviors on silicone. Although researchers typically consider the material to be relatively inert, the substrate's ability to adsorb matrix proteins can provide mechanosensory cues for unexpected bioactivity. Cheng et al. showed that liquid silicones used for syringe lubrication could also activate rigidity-associated responses and credited surface stress to be a contributing factor amongst many factors, to physically direct the observed cell responses. The team propose further investigations to understand how mechanical stimulation may contribute to material surface-associated pathologies including inflammation, granuloma formation and rare diseases such as BLA-ALCL. Future studies can potentially consider if rigidity responses to the silicone shell of an implant can cause capsular contraction, to effectively solve a significant complication of implants. The findings support further investigations on the surface stress of silicone materials during their design and application in medical devices.
More information: Zhu Cheng et al. The surface stress of biomedical silicones is a stimulant of cellular response, Science Advances (2020). DOI: 10.1126/sciadv.aay0076
Ovijit Chaudhuri et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity, Nature Materials (2015). DOI: 10.1038/nmat4489
Lindsay Keith et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma, Baylor University Medical Center Proceedings (2018). DOI: 10.1080/08998280.2017.11930221
Journal information: Science Advances , Nature Materials
© 2020 Science X Network