February 3, 2020 feature
Shape-morphing living composites
In a recent study published on Science Advances, L. K. Rivera-Tarazona and a research team in the departments of bioengineering and biological sciences at the University of Texas, Dallas, U.S., established a new method to explore genetic networks in the lab. They created living synthetic composites that changed shape in response to specific biochemical and physical stimuli. For example, Baker's yeast embedded in hydrogel can create a response material where cell proliferation (cell growth) caused controlled increase in the composite volume up to 400 percent. Genetic manipulation of the yeast permitted composites whose volume changed on exposure to L-histidine at a 14-fold higher rate than when exposed to D-histidine or other amino acids. The research team encoded an optogenetic (light responsive) switch in the yeast to control shape changes in space and time, with pulses of dim blue light at 2.7 mW/cm2. The living shape-changing materials can act as sensors and medical devices to respond to highly specific cues in the biological environment.
Materials that change shape can assist mechanical activity in a variety of devices including smart garments, sensors, microfluidics or drug delivery platforms. Traditional actuators such as solenoids are too large, heavy or power intensive to be used in such devices. Scientists can trigger shape change in synthetic polymers and gels using temperature, electric fields or chemicals, where the specificity of the response is dictated and limited by the physical character of the material.
Combining synthetic materials and the responsive nature of living organisms is a powerful strategy to allow multifunctionality in a single material. Examples include gels that self-heal through photosynthesis, wearable fluorescent biosensors and ethanol-producing three-dimensional (3-D) printed hydrogels. Yet these materials are unable to exert mechanical responses to environmental cues. Bioengineers had previously created mechanically active living composites with a bilayer of an elastomer and mammalian muscles to contract and relax like a muscle. There is still an existing need to create a strategy that unites cell growth to control the shape change of synthetic materials.

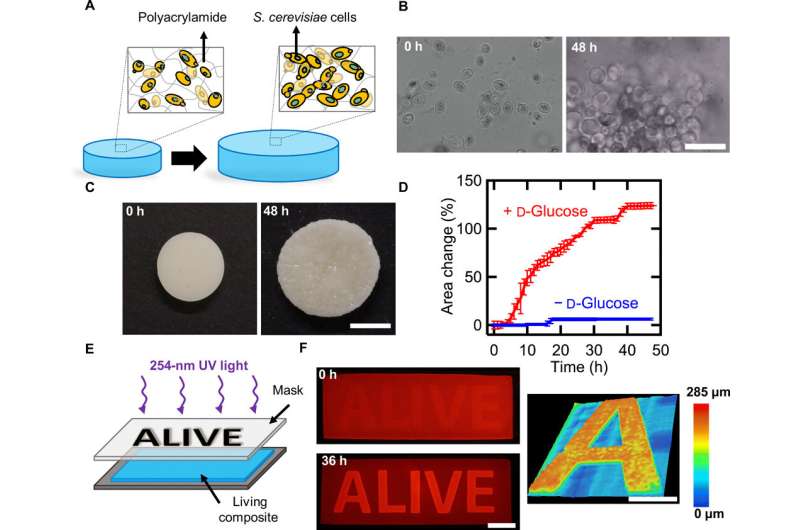
In the present work, Rivera-Tarazona et al. detailed hybrid materials containing living Saccharomyces cerevisiae (Baker's yeast or brewer's yeast) embedded into a polysaccharide hydrogel to proliferate in response to a combination of cues in the environment and induce shape changes in the composite. The team controlled cell loading, cell growth within the composite and hydrogel stiffness to control the magnitude of volume changes in the monolith. Yeast (S. cerevisiae) is an ideal and versatile platform to enable genetic engineering conditions and the scientists further harnessed shape changes to engineer microfluidic channels that selectively responded to fluids flowing through the microchannel.
S. cerevisiae can thrive in solid matrices that are much stiffer than hydrogels and survive a wide-range of conditions. Rivera-Tarazona et al. observed the stiff cells to proliferate within a soft hydrogel matrix and increase in volume, they credited the volume change to local displacement of the hydrogel due to cell proliferation. To quantify the effect of proliferation on macroscopic volume changes, they incubated the composites in yeast extract peptone D-glucose (YPD) nutrient media for 48 hours and patterned cell viability using ultraviolet (UV) light for the region of viable cells to arrange in the form of "ALIVE." The experiments showed how yeast proliferation caused volume changes within hybrid living matrices.
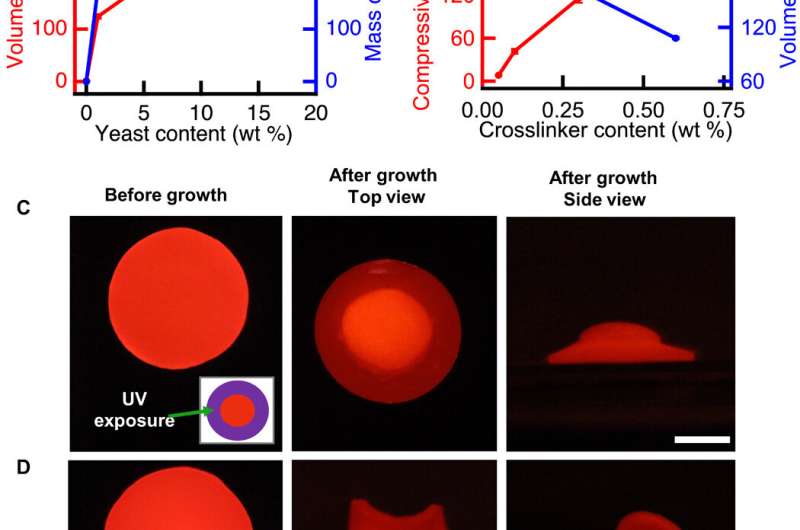
The bioengineers controlled the initial composition of the living composites to tune cell proliferation (growth)-driven shape change. The shape of the composites remained stable for more than 128 days in deionized water. The growing composites can provide opportunities to produce materials directly from renewable feedstocks and other unconventional resources. The scientists controlled volume change to create programmed composites morphing from 2-D to 3-D. When they used UV light to kill cells in programmed areas, they observed an absence of shape change in the composite. When they incubated the cell constructs in the nutrient medium YPD, the proliferation induced 2-D to 3-D transformation.
To program the shape change of living cells, the team genetically manipulated S. cerevisiae, a model eukaryote commonly used for heterologous protein expression. Rivera-Tarazona et al. used an L-40 yeast strain deficient in L-histidine; to prevent proliferation in the absence of L-histidine. For example, when they incubated the material films in media lacking L-histidine, they did not observe an effect on the shape of the composite, but in a medium containing L-histidine, these composites morphed to form a helix.
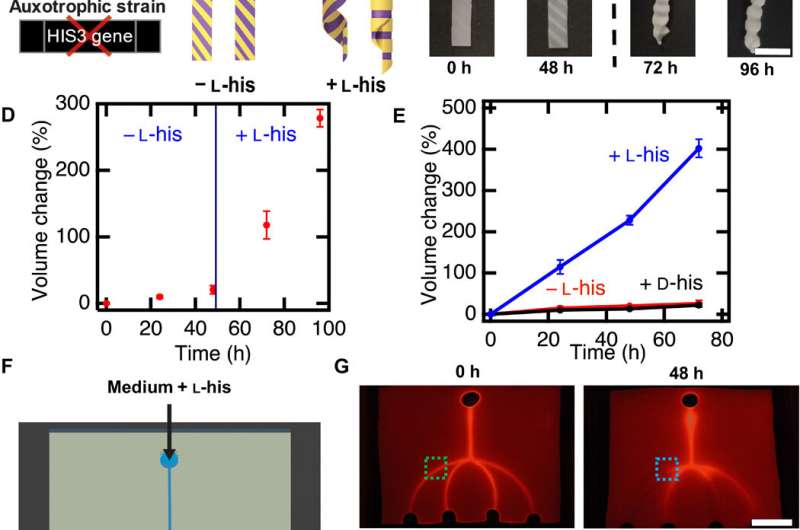
The bioengineers next fabricated microfluidic devices using living composites, to respond with specificity to fluid flowing through the channels within the living composite. In this setup, they first rendered all cells inviable using UV radiation, except for cells within the two microfluidic channels. Then using a fluorescent fluid, they visualized the performance of these devices. For instance, in the presence of L-histidine in the flowing medium, the two microchannels with viable cells grew in volume and blocked the channels, while the other channels remained open. In contrast, devices exposed to medium lacking L-histidine remained open due to lack of cell growth. These strategies can therefore facilitate smart microfluidic devices to form biosensors and replace traditional sensors or actuators.
The team then engineered optogenetic (light sensitive) switches into yeast to establish photoresponsive composites to spatiotemporally (relating to both space and time) control the shape changes. For this, they generated an experimental yeast strain to express a photoresponsive transcriptional switch and induced gene expression after illumination with blue light (455 nm). In the presence of light, they activated the HIS3 gene for cell proliferation in the absence of L-histidine.
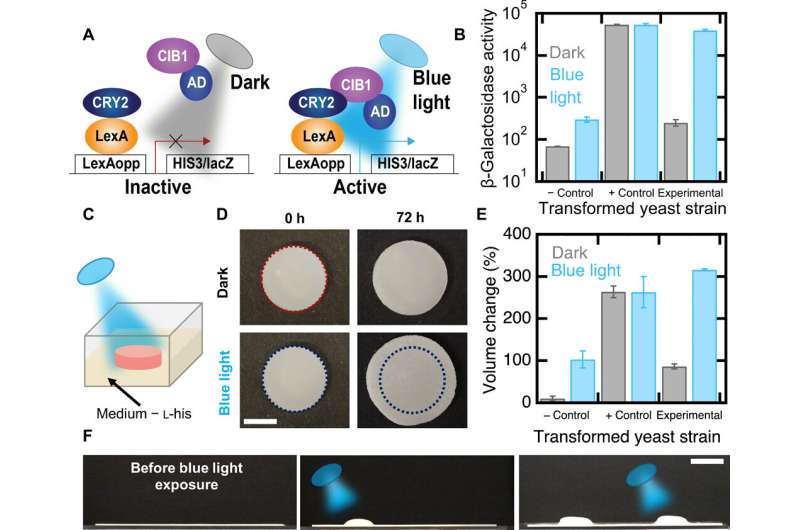
They also genetically engineered two additional yeast strains, a positive control that did not require light to activate HIS3 and a negative control lacking the CIB1 protein and is therefore auxotrophic (unable to synthesize a specific nutrient compound) for L-histidine in the presence or absence of blue light. The experimental strain showed higher rates of metabolic activity when exposed to light, while the negative control strain had low metabolic activity under blue light and also in the dark, whereas the positive control presented high metabolic activity under both conditions—as expected.
In this way, L. K. Rivera-Tarazona and colleagues combined patterned cell viability and patterned light illumination to provide spatiotemporal control of complex shape changes in living composites made of yeast transformed with an optogenetic switch. The living composites underwent cellular proliferation-induced shape changes and the scientists controlled the changes using the initial composition of the composite, or by patterning regions of viable cells. They engineered materials that capitalized on the genetic control of biological mechanisms such as cellular proliferation to establish responsiveness either in material shape or topography—relative to specific environmental cues. The findings will help research teams develop a host of devices ranging from drug delivery platforms to environmental sensors.
More information: Xinyue Li et al. Proceedings of the National Academy of Sciences. L. K. Rivera-Tarazona et al. Shape-morphing living composites, Science Advances (2020). DOI: 10.1126/sciadv.aax8582
Angelo Cangialosi et al. DNA sequence–directed shape change of photopatterned hydrogels via high-degree swelling, Science (2017). DOI: 10.1126/science.aan3925
Xinyue Liu et al. Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1618307114
Journal information: Science Advances , Proceedings of the National Academy of Sciences , Science
© 2020 Science X Network