New catalysts remove nitrogen oxide pollutants at lower temperatures
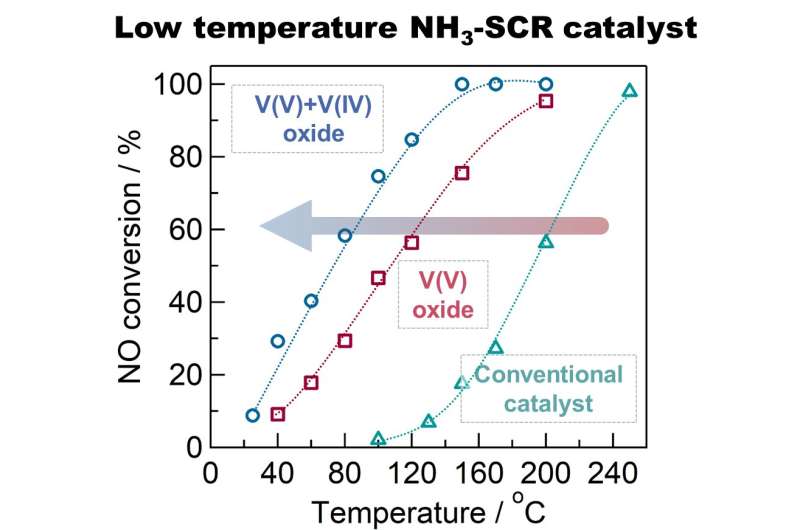
Scientists from Tokyo Metropolitan University have developed a low-temperature catalyst for removing NOx gas from industrial exhaust using ammonia. Composed of bulk "defective" vanadium oxide instead of vanadium oxides supported on titanium oxide as in commercial catalysts, the catalyst works at lower temperatures (< 150 degrees Celsius) with much higher efficiency. The team demonstrated a clear improvement in performance, and identified the reaction mechanisms responsible for the difference.
Nitrogen monoxide (NO) and nitrogen dioxide (NO2), or nitrogen oxides (NOx), are common atmospheric pollutants created by burning fossil fuels, coal and natural gas. They are a major cause of photochemical smog and acid rain, which makes their removal from vehicle and factory emissions extremely important. A key technology for removing nitrogen oxides is their reaction with ammonia via selective catalytic reduction (SCR), whe re NOx is rendered harmless via reduction to nitrogen and water. In particular, vanadium oxides supported on titania are known to have excellent selectivity for conversion to nitrogen, and have been successfully applied to stationary boilers.
However, a significant bottleneck for supported catalysts is the high temperature required for catalytic activity, often 200 to 400 degrees Celsius. This often results in units being placed close to e.g., the boiler in power plants, where they can be easily damaged not only physically by ash, but by the accumulation of ammonium sulfates. These deactivating factors can be avoided if the unit is placed downstream after an electrostatic precipitator for removing dust and a desulfation system to remove sulfate deposits. However, this approach requires high catalytic activity at lower temperatures, since the temperature of the exhaust gas has generally dropped to around 100 degrees Celsius by this point. A catalyst is needed that works at lower temperatures.
Now, a team led by Yusuke Inomata and Toru Murayama from Tokyo Metropolitan University have developed a catalyst based on bulk vanadium oxides. Vanadium (V) oxide (V2O5) is a common state of vanadium oxide; the team however successfully synthesized a mixture of vanadium (V) and vanadium (IV) oxides, or "defective" vanadium oxide, by heating a precursor to 270 degrees Celsius. They found that this "defective" catalyst had excellent catalytic activity at temperatures down to 100 degrees Celsius; at this temperature, the speed at which NOx is converted to harmless nitrogen was 10 times faster than conventional titania supported vanadium oxide catalysts, showing exceptional performance where conventional catalysts fall short. The improvement was attributed to the presence of V(IV) which creates "Lewis acid" (electron-accepting) sites, promoting the reaction of nitrogen oxide with ammonia to become nitrogen.
Beyond practical application to industrial catalysis, the team hope that the mechanisms they have uncovered serve as a model system for further scientific studies.
More information: Yusuke Inomata et al, Bulk Vanadium Oxide versus Conventional V2O5/TiO2: NH3–SCR Catalysts Working at a Low Temperature Below 150 °C, ACS Catalysis (2019). DOI: 10.1021/acscatal.9b02695
Journal information: ACS Catalysis
Provided by Tokyo Metropolitan University