Scientists propose a dynamic charge and oxidation state for single-atom catalysts
Pollutants coming out of cars' exhausts are harmful to the environment and public health. With the goal of overall curbing car emissions, the US Department of Energy (DOE) issued a challenge to scientists worldwide: catalytically converting 90 percent of all critical pollutants (hydrocarbons, CO2, NOx etc.) in car exhaust into less harmful substances at 150ºC. However, nanoparticle based heterogeneous catalysts—like the three-way exhaust catalyst used in cars—work best at high temperatures (between 200 and 400ºC), thus making the 150ºC DOE challenge seem difficult to attain.
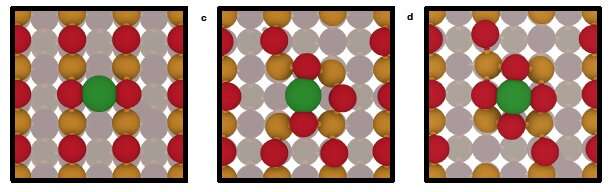
Now, researchers from the López Group, have studied in detail the behavior of Pt single atoms supported on CeO2—what the researchers argue would outperform the Pt nanoparticles supported on CeO2 currently employed in the three-way exhaust catalyst. The results, published in Nature Materials, show that the common assumption of a static charge in Single-Atom Catalysis is oversimplified. Instead, the scientists propose a dynamic charge, able to explain the unique reactivity found for activated single platinum atoms on ceria, which in turn can perform CO-oxidation meeting the DOE 150ºC challenge for emissions.
Dynamic charge and oxidation state
Since Single-Atom Catalysis field flourished, scientists have been working to understand the intimate behavior at the interface between Single-Atom Catalysts and the oxides supporting them, hoping this knowledge will allow the tuning of their catalytic activity. The scientists from the López Group combined Density Functional Theory (DFT) and first-principles Molecular Dynamics (BOMD) to elucidate what is exactly going on at the interface.
The simulations revealed a metastable system where the Pt atoms have several overlapping oxidation states, allowing the catalyst to shift from one state to another. These dynamically interconnected oxidation states are "a completely new concept," as Nathan Daelman, first author of the study, explains.
For the scientists, it's clear the dynamic behavior influences the reactivity of the system and, for the first time, they have been able to explain the Pt activation step needed for the three-way exhaust catalysts to properly function under DOE 150ºC working conditions. To the researchers, the next steps will be working to prepare a model of the mechanism that will be able to predict with temperature the behavior of the catalytic system.
More information: Dynamic charge and oxidation state of Pt/CeO2 single-atom catalysts, Nature Materials (2019). DOI: 10.1038/s41563-019-0444-y , nature.com/articles/s41563-019-0444-y
Journal information: Nature Materials
Provided by Institute of Chemical Research of Catalonia