New software brings lower-resolution cryo-EM maps into focus
Cryo-electron microscopy is now the most popular method for determining protein structures, which helps researchers develop drugs for different kinds of ailments. Over the last several decades, it has replaced X-ray crystallography because it can image proteins that can't easily be formed into large crystals. The new technique was so revolutionary that it won its developers the 2017 Nobel Prize in chemistry.
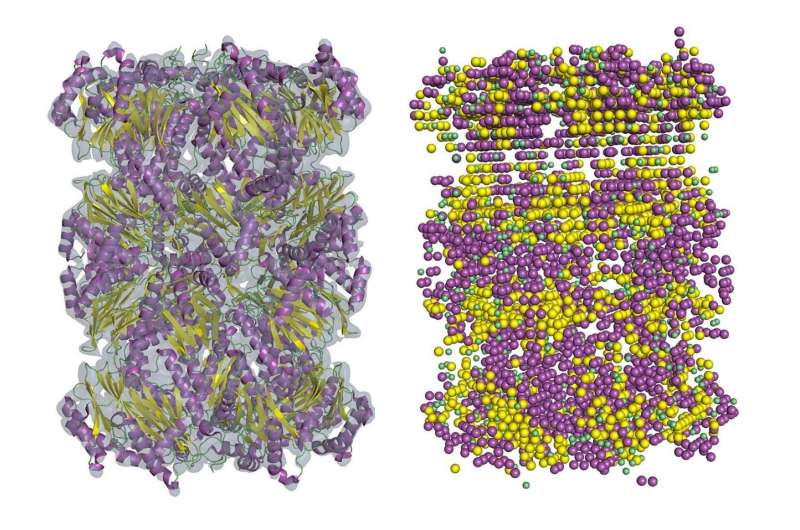
The final product of cryo-EM is a map of the density of atoms in biological molecules, but to achieve the level of detail researchers need, they need to conduct further analysis. A new study in the journal Nature Methods outlines a technique to bring low-resolution maps up to par.
The approach researchers use to do this depends on the level of detail they start with. Maps at 2 to 3 ångström (Å, a unit of length used to express the size of atoms and molecules) are generally considered high-resolution. However, maps of this quality are difficult to achieve, and many are still commonly produced in the range of 4 to 10 Å. Of all the proteins deposited to the Electron Microscopy Data Bank from 2016-18, more than 50% were solved at intermediate resolution.
"If the resolution is better than three, then conventional tools can trace amino acid position and build a map of atom positions. But frequently cryo-EM cannot give you a 3 Å map," said Daisuke Kihara, a professor of biological sciences and computer science at Purdue University. "In maps of 5 Å or lower, you usually can't see chain connectivity at all."
Proteins are actually chains of amino acids, and bonding between amino groups and carboxyl groups sometimes creates certain patterns of folding. These patterns, known as alpha helices and beta strands, form the secondary structure of the protein.
In maps from 5 to 8 Å, some fragments of the secondary structure of proteins are usually visible, but tracing the entire chain would be very difficult. Kihara's new method, known as Emap2sec, uncovers secondary structures in maps from 6 to 10 Å.
Emap2sec has a deep convolutional neural network at the core of its algorithm. These networks are deep-learning systems primarily used to classify images, cluster them by similarity and perform object recognition. It works for protein structure identification in 3-D maps because the method "convolves" local map density features to images of a larger region as the information passes through layers of neural network. The local prediction is made in the context of a large region of the map.
Identified secondary structures in 3-D maps help researchers to assign known structures of proteins that have already been solved into the map. This means they sometimes have a starting point, or at least a clue of what some of the structure looks like. Emap2sec can help researchers fit their piece into the puzzle more quickly and easily. The identified structure information might also be helpful in finding errors in structure modeling.
More information: Sai Raghavendra Maddhuri Venkata Subramaniya et al. Protein secondary structure detection in intermediate-resolution cryo-EM maps using deep learning. Nature Methods (2019) DOI: 10.1038/s41592-019-0500-1
Journal information: Nature Methods
Provided by Purdue University