We finally understand how oxygen reacts on platinum
Platinum is a widely used catalyst, but its precise mechanism largely remains a mystery to scientists. Ludo Juurlink has now demonstrated for the first time how oxygen reacts on the platinum surface. Together with Ph.D. students Kun Cao and Richard van Lent and international colleagues, he has published his findings in PNAS.
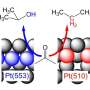
Earlier this year, Juurlink solved a 40-year-old problem in chemistry, together with Richard van Lent and the DIFFER institute. Using a unique curved platinum surface, he proved how hydrogen reacts on platinum. In his current research, he again used the curved platinum, this time investigating the reaction with oxygen.
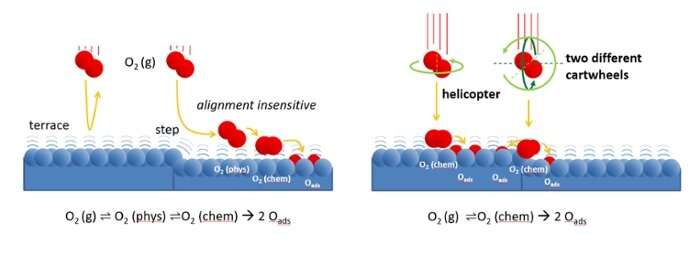
This has led to an interesting discovery. Juurlink and colleagues observed that oxygen reacts on platinum in a different way than the much lighter hydrogen. The curved platinum was again crucial for this discovery. "Because the platinum surface is curved, the atomic structure changes very gradually along the surface," explains Juurlink. This structure can be compared to a staircase with steps that become increasingly narrow toward the edges. In the middle, the surface looks more like a ballroom."
The reactivity of hydrogen turned out to depend on how close the steps of the catalyst are to each other. This is also the case with oxygen, but for a fundamentally different reason. "The steps have a different effect on oxygen than on hydrogen."
According to Juurlink, this mainly has to do with the larger mass of oxygen. "Because oxygen is heavier than hydrogen, the interaction with the platinum surface starts from a greater distance," he says. "The oxygen molecule already feels the interaction with platinum, but cannot yet see the details. As a result, the reaction takes place in a different way than with hydrogen."
For the experiment, it was necessary to control the direction of rotation of the oxygen molecules. This required cooperation with a Japanese colleague, Mitsunori Kurahashi, who built a unique device for this purpose. "Last year, I had the opportunity to carry out measurements in his lab for two weeks on a grant from the institute where Kurahashi works," says Juurlink.
"This is a beautiful fundamental discovery, concludes Juurlink, which may also have an impact on existing applications. The reaction of oxygen to platinum is essential in the sustainable energy sector and in improving air quality. "For example, the reaction takes place in hydrogen fuel cells and in car exhaust systems," says the chemist. "The fact that we can now measure how the reaction proceeds at such a detailed level, poses challenges to theoretical models that describe this chemical reaction and make predictions about it."
More information: Kun Cao el al., "Steps on Pt stereodynamically filter sticking of O2," PNAS (2019). www.pnas.org/cgi/doi/10.1073/pnas.1902846116
Journal information: Proceedings of the National Academy of Sciences
Provided by Leiden University