A new mechanism helps explain differences between eukaryotic and bacterial proteomes
Why do distinct species have different proteins? Is there a key that allows eukaryotic cells to produce proteins involved in multicellularity that are mostly absent in prokaryotes? These are some of the questions addressed by the team of ICREA researcher Lluís Ribas, group leader of the Gene Translation lab at the Institute for Research in Biomedicine (IRB Barcelona). Their work has led to the discovery of a mechanism that allows eukaryotic cells to synthesize proteins that bacteria find hard to produce.
Published recently in the journal Molecular Biology and Evolution, the study has been done in collaboration with the team headed by Iñaki Ruiz-Trillo, ICREA researcher at the Institute of Evolutionary Biology in Barcelona.
"We discovered and described a mechanism evolved in eukaryotes that facilitates the synthesis of large, unstructured proteins such as those present in the extracellular matrix. Those are the proteins that surround each cell and allow them to associate and communicate with their environment," says Ribas.
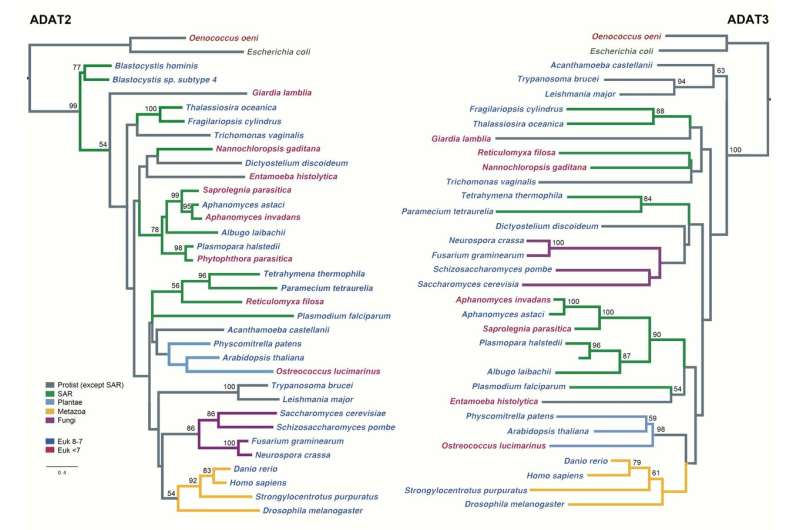
The results of the research explain that the emergence of this functional improvement in some transfer RNAs (tRNA) facilitated the synthesis of proteins highly enriched in a specific set of amino acids, and drove an enrichment of genes coding for these tRNAs in eukaryotic genomes.
Many of these proteins are highly relevant to human health, and understanding the mechanisms essential to their synthesis may allow the development of strategies to inhibit their production in those diseases caused by their overabundance.
More information: Àlbert Rafels-Ybern et al. The expansion of Inosine at the wobble position of tRNAs, and its role in the evolution of proteomes, Molecular Biology and Evolution (2018). DOI: 10.1093/molbev/msy245
Journal information: Molecular Biology and Evolution