Molecular details of protein reveal glimpse into how kidney stones form
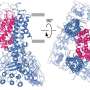
Kidney stones—solid, pebble-like grit that forms when too much of certain minerals like calcium are in the urine—can strike men, women, and increasingly, children, and the presence and pain of stones afflicts more than 12 percent of the world's population. Using the 2017 Nobel Prize-winning technique of cryo-electron microscopy (cryo-EM) to capture a high-resolution image of an ion channel protein, called TRPV5, that removes calcium from urine, researchers from the Perelman School of Medicine at the University of Pennsylvania, Rutgers University Medical School, and Temple University, found fresh clues as to how kidney stones form.
With new information gained from TRPV5's molecular structure, researchers will now be able to use bioinformatics to discover compounds that interact with TRPV5 to treat and prevent kidney stones in at-risk populations. The team published their findings in Nature Communications.
Led by Vera Moiseenkova-Bell, Ph.D., an associate professor of Systems Pharmacology and Translational Therapeutics, the team captured an image of the TRPV5 ion channel protein in both an open and closed state. All cells have channels in their outer membranes that allow for the flow of small ions such as sodium, potassium, or calcium. This two-way movement aids in many roles for example, triggering an immune response, communicating between brain cells, and filtering by the kidney.
Close to 99 percent of calcium is reabsorbed by kidney tubules, and TRPV5 is only made in the cells that line tubules where calcium level in the urine is maintained. Most kidney stones contain calcium, and too much calcium in urine predisposes people to the formation of these painful deposits.
Cryo-EM uses an electron beam to take thousands of snapshots of individual frozen protein molecules. Algorithms then combine the multiple images to sharpen the overall picture of a molecular structure. Using these images, Moiseenkova-Bell, who is also director of Penn's Beckman Center for Cryo-Electron Microscopy, and her team revealed the TRPV5 structure to answer questions about the protein's physiological role in disease.
"We were able to see, for the first time, how this protein opens by activating membrane lipids," said co-first author Taylor Hughes, a graduate student in Moiseenkova-Bell's lab. "Many proteins are regulated in a similar way, so our structure lays the groundwork for understanding this process in other settings."
Postdoctoral fellow and co-first author Ruth Pumroy, Ph.D., adds that the team also discovered the structure of a closed channel in the presence of a protein called calmodulin, which directly plugs the pore of the channel without causing the pore to move. "This revealed a unique mechanism of TRPV5 inhibition which could be useful for finding novel binding partners and drug discovery," said Pumroy.
Rutgers coauthor Tibor Rohacs and co-first author Aysenur Yazici, a graduate student in his lab, verified predictions of how the channel works by changing individual amino acids in the TRPV5 structure to see if the flow of calcium through the altered channel would differ. When amino acids in contact with a lipid in the TPRV5 structure were altered, TRPV5 did not allow calcium to flow into the cell. When another TPRV5 amino acid was changed in the channel, the inhibitory effect of calmodulin disappeared. The collaborators at Temple used sophisticated computer programs to further validate the findings.
Journal information: Nature Communications