Hybrid catalyst with high enantiomer selectivity
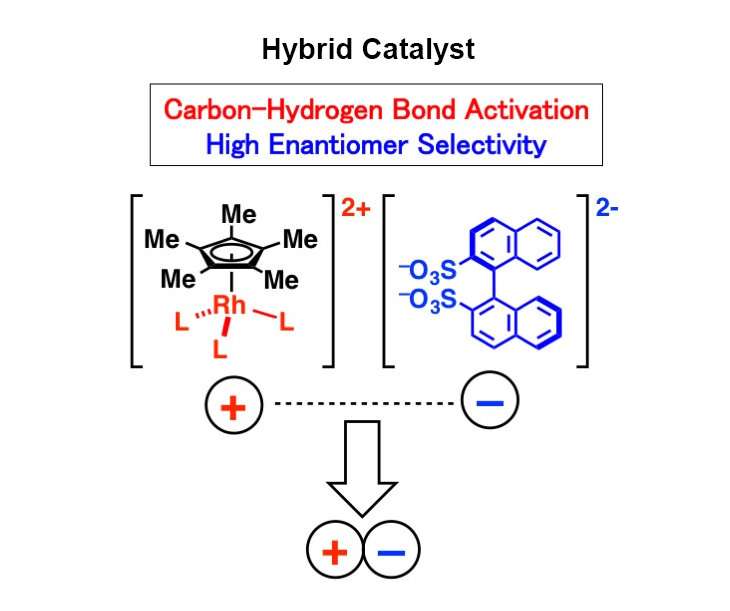
A group of Japanese researchers has developed a technology to create a hybrid catalyst from simple-structured, commercially available rhodium and organic catalysts, which reduces chemical waste and produces molecules with high selectivity of an enantiomer, a pair of molecular structures that are non-superimposable mirror images of each other. This technology is expected to assist in rapid and low-cost drug synthesis.
The technology was developed by scientists including Professor Shigeki Matsunaga and Assistant Professor Tatsuhiko Yoshino, both of Hokkaido University's Faculty of Pharmaceutical Science, and Professor Kazuaki Ishihara and Associate Professor Manabu Hatano, both of Nagoya University's Graduate School of Engineering.
The two molecular structures found in an enantiomer have different effectiveness when used as drugs, even though their chemical properties are similar. One molecular structure can be effective, while the other can trigger serious side effects. It is therefore important to select the desired molecular structure for chemical conversion when synthesizing drugs. In addition to manufacture medicines with less waste, it is necessary to have the chemical conversion occur only at a desired carbon-hydrogen bond with the use of catalysts. To fulfill these two requirements, scientists have been using expensive rhodium catalysts made in complex, multi-phased production processes. The limited availability of such rhodium catalysts has made it difficult to apply them for industrial use.
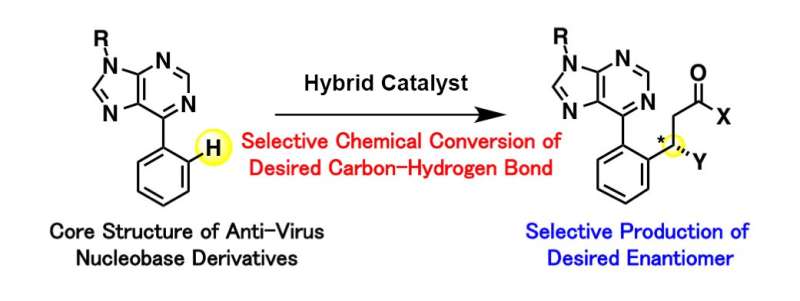
In the present study published in Nature Catalysis, simple-structured, commercially available rhodium was combined with a readily available organic catalyst in one step by utilizing ionic interactions. A simple rhodium catalyst is capable of activating the desired carbon-hydrogen bond, but it's not good at selectively obtaining only one molecular structure in an enantiomer. Organic catalysts, meanwhile, are capable of producing the targeted molecular structure, but are not effective in activating the desired carbon-hydrogen bond. This newly developed hybrid catalyst is able to compensate for both individual shortcomings. Using the hybrid catalyst, the researchers succeeded in activating only the targeted carbon-hydrogen bond and selectively obtaining one molecular structure in the enantiomer when conducting chemical conversions of nucleobase derivatives, which is expected to boost antiviral performance.
"The technology is highly versatile because a variety of organic catalysts can be combined with the simple rhodium catalyst," says Shigeki Matsunaga. "It is expected to help make core chemical structures for nucleotide medicine, which is gaining attention as a next-generation medicine to treat a number of conditions cheaply and in an environmentally friendly way."
More information: Shun Satake et al. Pentamethylcyclopentadienyl rhodium(III)–chiral disulfonate hybrid catalysis for enantioselective C–H bond functionalization, Nature Catalysis (2018). DOI: 10.1038/s41929-018-0106-5
Journal information: Nature Catalysis
Provided by Hokkaido University