Efficient, eco-friendly production of fine chemicals
The chemical industry produces not just valuable vitamins, pharmaceuticals, flavours and pesticides, but often a large amount of waste, too. This is particularly true of pharmaceutical and fine-chemical production, where the volume of desired product may be just a fraction of the volume of waste and unsaleable by-products of synthesis.
One reason for this is that many chemical reactions make use of catalysts in dissolved form, as Javier Pérez-Ramírez, Professor of Catalysis Engineering, says. Catalysts are substances that accelerate a chemical reaction. In the case of dissolved catalysts, it often takes a huge amount of effort to separate them from the solvent and from the reaction products for reuse. Catalysts in solid form avoid this problem altogether.
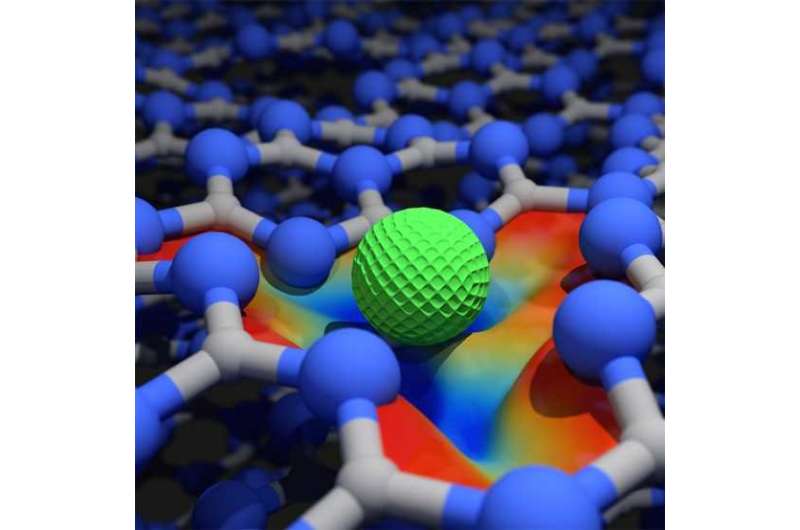
Pérez-Ramírez and his group have now collaborated with other European scientists and an industry partner to develop just such a solid catalyst for a major chemical reaction, as the researchers report in the magazine Nature Nanotechnology. Their catalyst is a molecular lattice composed of carbon and nitrogen atoms (graphitic carbon nitride) that features cavities of atomic dimensions into which the researchers placed palladium atoms.
Efficient catalyst for a Nobel-prizewinning reaction
By making tiny particles of this palladium-carbon-nitrogen material, the scientists were able to show that it catalyses what is known as the Suzuki reaction very efficiently. "In chemistry, forming a bond between two carbon atoms is often done using the Suzuki reaction," says Sharon Mitchell, a scientist in Pérez-Ramírez's lab. It was this reaction that won Japanese scientist Akira Suzuki and two colleagues the Nobel Prize in Chemistry 2010.
Thus far, the process in commercial scale has widely used soluble palladium catalysts. Earlier attempts to attach the soluble catalyst to a solid body always resulted in relatively unstable and inefficient catalysts.
Considerably less waste
The ETH researchers' new palladium catalyst is much more stable. For that reason, and because it does not dissolve in the reaction liquid, it can be used over a much longer time period. What's more, the catalyst is much more cost-effective and around twenty times more efficient than the catalysts used today.
"That means the new catalyst not only cuts the costs of synthesising fine chemicals, it also reduces the consumption of palladium and decreases the amount of waste," Pérez-Ramírez says. The catalyst might soon be ready for use in industry: the scientists claim that it should be easy to scale up catalyst production and use from the laboratory.
As the researchers point out, the use of graphitic carbon nitride as a solid catalyst is not limited to the Suzuki reaction. It should also be possible to populate the lattice with atoms of metals other than palladium in order to catalyse other syntheses. The ETH scientists will explore these possibilities in future research. They also plan to found a spin-off company to market this novel family of catalysts.
More information: Zupeng Chen et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling, Nature Nanotechnology (2018). DOI: 10.1038/s41565-018-0167-2
Journal information: Nature Nanotechnology
Provided by ETH Zurich