Computing catalysts—team unlocks the molecular secrets to a popular polymer
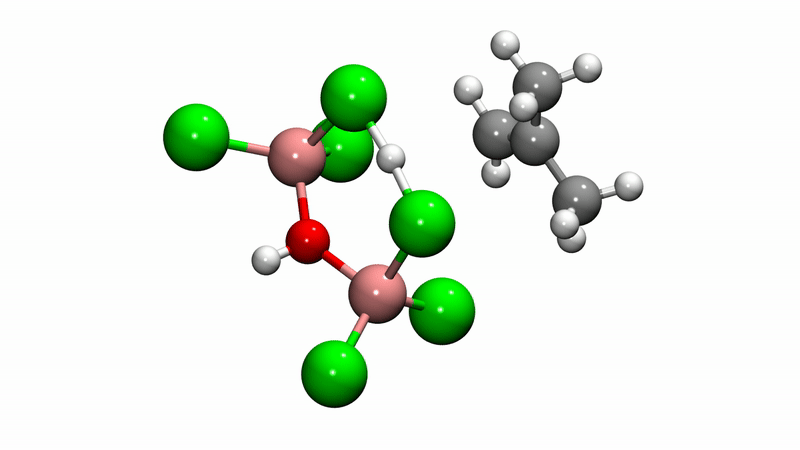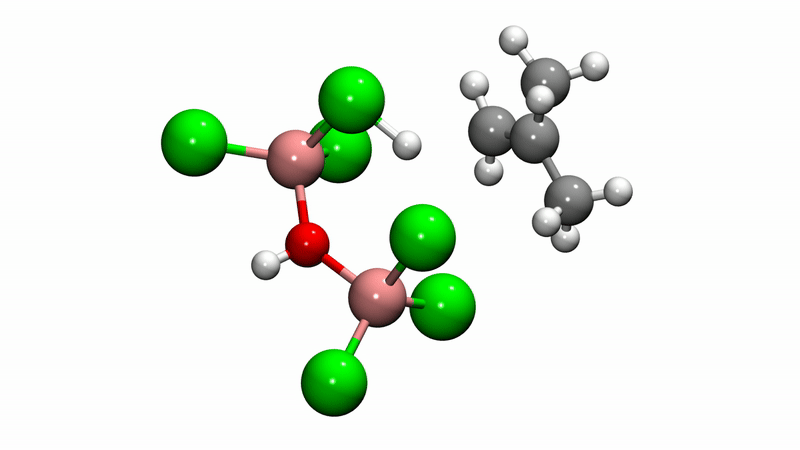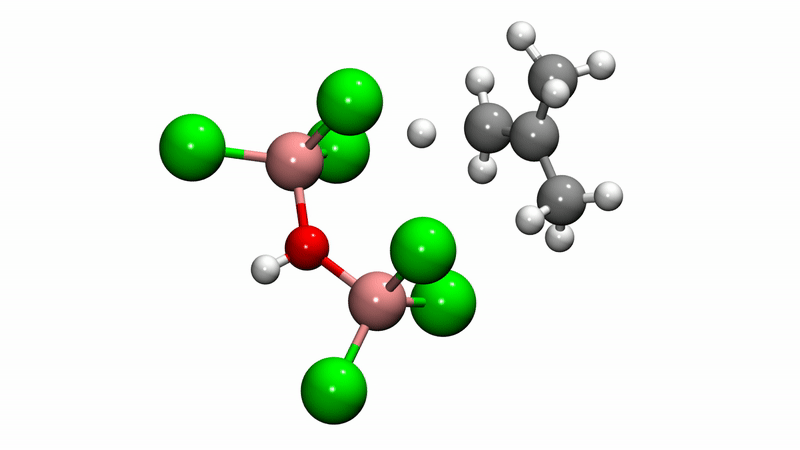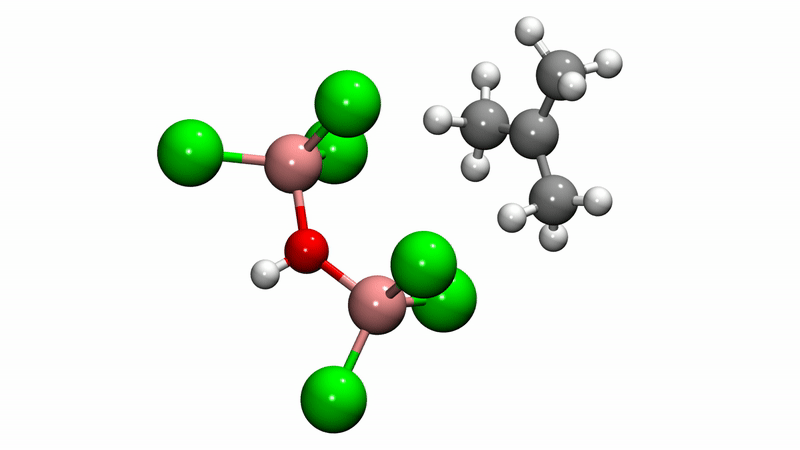
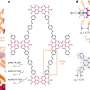
Polyisobutylene (PIB) is a workhorse polymer that is found in a multitude of products, ranging from chewing gum, to tires, to engine oil and gasoline additives. Although commercially produced in large quantities since the 1940s, PIB chemistry was a mystery—scientists weren't sure how the reaction mechanism that creates the polymer happens at the molecular level, which limited further potential.
However, a collaboration between the University of Pittsburgh's Swanson School of Engineering and Wickliffe, Ohio-based Lubrizol Corporation has unlocked the secrets of PIB's reaction mechanism. The group's findings were published this month in the journal ACS Catalysis.
Principal investigator is Karl Johnson, the William Kepler Whiteford Professor in the Swanson School's Department of Chemical & Petroleum Engineering. Funding for the research was provided by Lubrizol, which in 2014 established a $1.2 million strategic partnership with the Department and Swanson School to jumpstart research innovation that also offers opportunities for undergraduates to participate.
"PIB is an incredibly versatile polymer. It can have many different properties depending on how it is made. There are many different 'recipes' for making PIB, each employing different catalysts and reaction conditions, but it turns out that no one really knows what is happening at the molecular level. Finding out what is going on is important because it is harder to control a process that you don't understand."
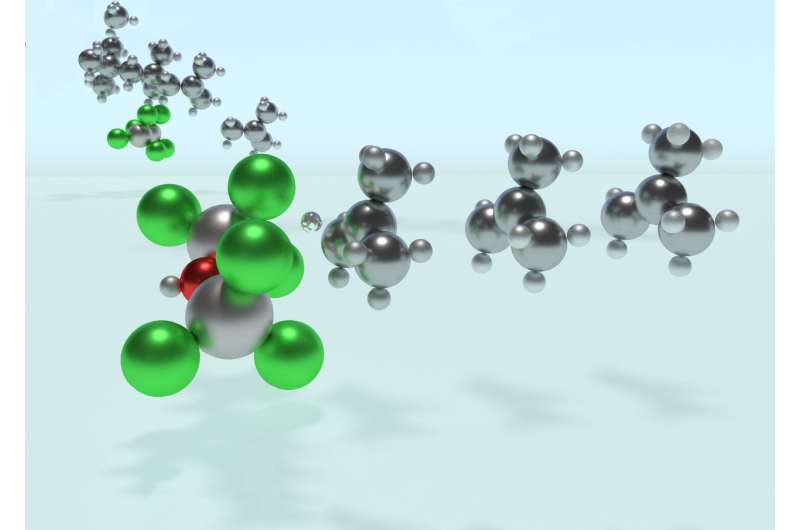
Solving this catalytic puzzle is of interest to Lubrizol, which specializes in ingredients and additives for polymer-based products. Utilizing the University's Center for Research Computing to analyze the molecular processes, the Pitt/Lubrizol group found that the assumed reaction mechanism was not correct and that initiation of the reaction requires a "superacid" catalyst.
"These findings provide fundamental insight into the PIB reaction mechanism that could potentially be used to design different catalysts and to control the reaction—and hence, the potential range of products—in ways that are currently not possible." Dr. Johnson said. "This project shows the value of creating academic/industrial partnerships to pursue research that might not be possible if pursued independently."
More information: Minh Nguyen Vo et al, Mechanism of Isobutylene Polymerization: Quantum Chemical Insight into AlCl3/H2O-Catalyzed Reactions, ACS Catalysis (2018). DOI: 10.1021/acscatal.8b01494
Journal information: ACS Catalysis
Provided by University of Pittsburgh