Designer molecule kills malarial parasites

A research team from ANU and The University of Queensland has designed and made a molecule derived from a human protein that kills the parasite which causes malaria.
Treatments for malaria, a disease that kills a person every 90 seconds, are becoming less effective because of drug resistance and there is a risk to the future control of the disease.
Lead researcher Associate Professor Brendan McMorran from ANU said the team was a step closer to developing a new and effective treatment for malaria.
"We have designed and created a small fragment of a human protein called Platelet factor 4, known as PF4, that can kill the microbial parasite that causes malaria, Plasmodium," said Dr McMorran from the John Curtin School of Medical Research.
"PF4, which my research group at ANU identified in previous work, is the only human protein known to kill malarial parasites."
The protein is relatively large and not suited to use as a treatment, so the team copied parts of the protein that are critical for terminating the malarial parasites into a smaller molecule, otherwise known as a peptide.
"We have shown that this peptide kills the parasite in a way that is completely different to how current antimalarial drugs work," Dr McMorran said.
"This is important because finding new ways to kill the parasite will help us overcome the drug-resistance problems."
The study involved expertise from several disciplines from the John Curtin School of Medical Research, the ANU Research School of Biology and The Institute for Molecular Bioscience at The University of Queensland.
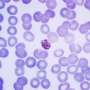
The researchers tested the peptide's effectiveness at killing the malarial parasite cultured in human red cells under laboratory conditions.
The team will develop more potent versions of the peptide that can be used as new treatments for malaria.
"To do this, we plan to modify the peptide to include new functions that target other vulnerable parts of the parasite, and make the peptide more suited to being administered to people," Dr McMorran said.
The research is published in Cell Chemical Biology.
More information: Nicole Lawrence et al. Defense Peptides Engineered from Human Platelet Factor 4 Kill Plasmodium by Selective Membrane Disruption, Cell Chemical Biology (2018). DOI: 10.1016/j.chembiol.2018.06.009
Journal information: Cell Chemical Biology
Provided by Australian National University