Avoiding catastrophe: Yeast study reveals clues to maintaining genome size
As cells divide, they must accurately split their DNA between the two daughter cells or risk having an uneven number of chromosomes which can lead to developmental disorders and cancer. A new Donnelly Centre study uncovers how a key molecular machinery drives this process and gives clues to why some children develop aggressive kidney tumours.
Led by Tina Sing, a Ph.D. student in Professor Grant Brown's lab in the Donnelly Centre and Department of Biochemistry, the study's findings are published in the June xx issue of The Journal of Cell Biology.
Brown likens the genome to an instruction book organized into a set number of chapters, or chromosomes. "It's important that the number of chapters stay constant," he says. "It would be bad if you lack instructions for certain processes but surprisingly it's also bad if you have too many instructions."
Having an extra copy of chromosome 21 leads to Down syndrome, while an absence of one X chromosome will turn females sterile as seen in Turner syndrome. In cancer cells, whole genome duplication followed by haphazard chromosome loss allows tumour cells to accumulate genes which help them outgrow healthy cells.
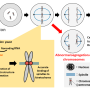
"When a cell makes a decision to divide it needs to make sure its DNA is equally segregated between both daughter cells," says Sing, who is now a postdoctoral researcher at the University of California, Berkeley. "The cells must first replicate their DNA and then they have to pull apart these two copies of the DNA so that each daughter cell receives one complete copy of the entire genome."
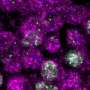
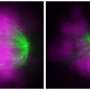
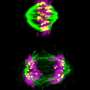
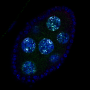
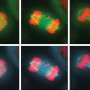
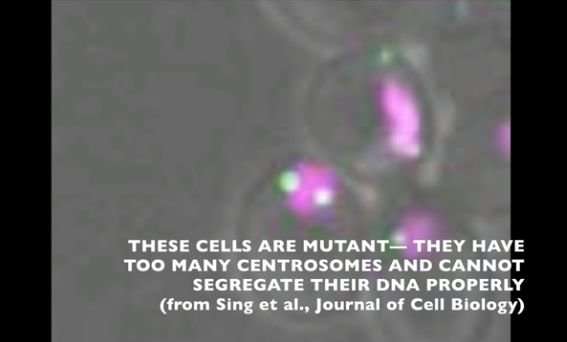
Sing uncovered a new role for a known protein machinery called RSC, for "remodels the structure of chromatin" and pronounced as "risk", in helping separate the duplicated chromosomes equally between daughter cells. RSC does this by helping the formation of the centrosome, a structure that sprouts tiny filaments which grab each set of chromosomes and pulls them apart. "We found that if cells lack RSC function then this causes abnormal DNA segregation and a spontaneous doubling of chromosome number in cells," says Sing.
In their experiments, Sing and Brown used budding yeast—the same single-celled microbe that helps bread rise and beer ferment—which showed uncanny resemblance to cancer cells when RSC is no longer working. Besides having a higher number of chromosomes, yeast cells lacking RSC function also have more centrosomes. Whereas heathy cells typically have two centrosomes during cell division, RSC mutants often had many more, making it difficult for them to segregate their chromosomes properly.
Previously, RSC was known for its role in switching genes on and off. Sing's finding that RSC is also important for DNA segregation was unexpected and ties together with previous findings from other labs to potentially help explain how a form of childhood cancer develops.
Mutations in the human version of RSC also lead to spontaneous increase in chromosome number and have been found in rhabdoid tumours, a highly aggressive form of kidney cancer. Also, if mouse cells lose RSC function, they can turn cancerous. However, there was nothing known about RSC that would suggest its involvement in chromosome segregation. Now, thanks to the insights from yeast cells, it is possible that RSC has a similar role in humans.
"It's always surprising to think about how fundamental processes in yeast also take place in humans," Sing says. "We think that our study gives some insight into this observation in cancer cells and we think it's possible that this complex is also helping with chromosome segregation in human cells."
Sing's finding was serendipitous. When she started working on RSC, her goal was to dig deeper into some of its more conventional roles. It was only after she repeatedly noticed that cells lacking RSC function had twice the normal number of chromosomes that she realized she was onto something unexpected. "The increase in chromosomes was more interesting than what we were looking for," she says. "I think the study is a good example of how sometimes when you set out to study a specific biological problem you can be surprised by the data that you find and sometimes just following your curiosity down a different path can lead to understanding another aspect of biology."
Journal information: Journal of Cell Biology
Provided by University of Toronto