How to make greener biofuels

The climate is under pressure. And despite a number of climate agreements, we still have not bent the curve on fossil fuel emissions, which continue to rise. One of the big sinners is the fuel we use for transport.
In Europe, burning of fossil fuels for transport contributes to 20 per cent of our collective emissions of greenhouse gases. In the USA this figure is 26 per cent.
Since we're not about to stop transporting ourselves and our goods any time soon, we clearly need to find a sustainable and CO2-neutral solution to meet these energy requirements.
One part of the solution will be to continue electrification of railways and single person transport, while road, sea, and air freight will probably continue to burn liquid fuels.
Renewable sources of biomass such as sugar cane, wood, or straw, have the potential to meet a significant fraction of these energy needs. The problem is however, that they are not particularly effective sources of energy. But here at the Technical University of Denmark (DTU), we may just have a solution.
Good and bad biofuel
Biomass such as corn, sugar cane, or vegetable oil, which are also food sources, are known as first generation biofuels. Apart from the ethical problems of turning food into fuel, they are also of limited potential in terms of limited crop yields per farming area.
Second generation biofuels are more climate-friendly. They use non-edible biomass such as wood, straw, and other farm waste. This biomass is composed of cellulose, hemicellulose, and lignin, which together provide structure and strength to plants.
Enzymatic and fermentation processes can convert cellulose and hemicellulose into alcohols, such as ethanol and butanol. These can be mixed with fossil fuels, for example gasoline, or further processed into hydrocarbons, which can be used for jet fuel.
The biological processes that transform biomass to alcohol yield a relatively low amount of energy, around 35 per cent of the potential energy stored within the biomass. So we are certainly not using this biomass to its full potential.
This low energy yield is due to the fact that the enzymes cannot convert lignin (which typically represents 25 to 35 per cent of the potential energy) to alcohol. It requires a lot of energy to break down the protected structure of lignin so that the enzyme can convert the cellulose.
The primary fermentation product is a thin watery alcohol solution, with an alcohol concentration similar to beer. This needs to be distilled into a clean alcohol by a process that also requires energy.
So the entire process of converting biomass to a usable biofuel requires a lot of energy and is not always optimal.
Production of biofuel by rapid heating
Rapid heating, or pyrolysis to give it its scientific name, is a process where the biomass is heated to 500-550 degrees centigrade in an oxygen-free atmosphere. This breaks down all of the biomass into an oil, and gas and coke residues. Coke is carbon from organic material, like the charcoal you use on your BBQ.
Bio-oil from conventional pyrolysis contains lots of water and organic molecules which also contain oxygen such as sugars, carboxylic acids, ketones, aldehydes, and phenols.
These molecules are not particularly stable and promote the formation of solid coke when heated. The 'raw' bio-oil is therefore not an optimal product to store as 'liquid energy.'
So we need one more step in the process.
Reacting the bio-oil with hydrogen, allows it to be converted into a mixture of gasoline and diesel by separating the oxygen as water. This is done under high pressure (up to 100 bar), moderate temperature (250 to 350 degrees centigrade), and with the help of a catalyst used in oil refineries.

Unfortunately, this has proved difficult to do in practice as the highly reactive oil creates coke when heated, which deactivates the catalyst, and clogs the reactor in which the hydrogen reaction takes place.
A two-in-one method
So what can we do? We have two methods, each of which have potential, but neither of them manages to turn biomass into biofuel efficiently.
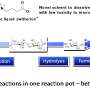
At DTU, we've combined the pyrolysis method and the catalyst reaction with hydrogen in a step called catalytic hydropyrolysis.
It works by feeding a reactor with solid biomass, while the catalyst is in constant motion. Hydrogen is fed in to the base of the reactor, partially lifting the biomass so it can move around. This allows the most reactive molecules in the bio-oil to react with hydrogen as soon as they form.
This prevents the molecules from reaching a state in which they could form coke, and so avoids deactivating the catalyst and removes around 95 per cent of the oxygen in the bio-oil.
This leaves the less reactive molecules (about five per cent), which can be removed by sending the oil through a traditional reactor, containing a catalyst, which is kept fixed.
The traditional reactor resembles those used to remove sulphur from petroleum in a process usually used in oil refineries to avoid sulphur in gasoline and diesel, thereby reducing sulphurous car emissions.
The reaction between hydrogen and biomass produces heat, so there is no need for additional energy during the process.
Distillation occurs automatically
The product of catalytic hydro-pyrolysis are oil and water, which as we all know, do not like to mix and therefore separate automatically. This is important as it saves on energy-heavy distillation, which is normally used to separate alcohol from water.
This process creates light gases and less coke waste. The lighter gases include methane, ethane, and propane, and significant amounts of carbon monoxide and CO2. The latter two can be further reacted with hydrogen to create methane, which together with other light hydrocarbons can be used as biogas, meaning even less waste from the original biomass.
Energy can be stored
Catalytic hydropyrolysis can be combined with other renewable energy technologies such as wind energy or solar power to produce the hydrogen, which is used in the catalytic hydropyrolysis process, via electrolysis of water. Here, you apply a current to water to separate hydrogen from oxygen. This would be especially useful when there is a surplus of renewable electricity.
Alternatively, hydrogen can be produced from the light gases. In this mode biogas is not generated as a bi-product. This makes catalytic hydropyrolysis a flexible technology, which can be used to store surplus electricity in liquid and gaseous fuels, or you could only use biomass and only produce liquid fuels.
Markedly better biomass yields
So how well does our method work?
So far we have been able to extract around 58 per cent of the potential energy of beech wood as bio-oil. This is significantly higher than the biological processes, which yield around 35 per cent of the energy from biomass.
We have also made a computer simulation, which shows that we could achieve energy yields of up to 87 per cent, when the electricity and biomass are converted to bio-oil and biogas.
Although biomass is renewable, it is still a limited resource. So it's important to produce biofuels as efficiently as possible. And so catalytic hydropyrolysis is an interesting technique that could produce better yielding biofuels in the future.
Provided by ScienceNordic
This story is republished courtesy of ScienceNordic, the trusted source for English-language science news from the Nordic countries. Read the original story here.