Super-resolution microscopy in both space and time
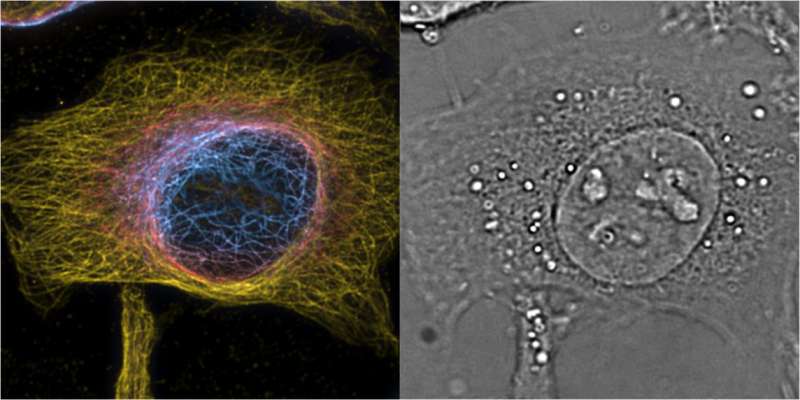
Super-resolution microscopy is a technique that can "see" beyond the diffraction of light, providing unprecedented views of cells and their interior structures and organelles. The technique has garnered increasing interest recently, especially since its developers won the Nobel Prize in Chemistry in 2014.
But super-resolution microscopy comes with a big limitation: it only offers spatial resolution. That might suffice for static samples, like solid materials or fixed cells, but when it comes to biology, things become more complicated. Living cells are highly dynamic and depend on a complex set of biological processes that occur across sub- second timescales, constantly changing. So if we are to visualize and understand how living cells function in health and disease, we need a high time (or "temporal") resolution as well.
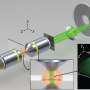
A team led by Professor Theo Lasser, the head of the Laboratory of Biomedical Optics (LOB) at EPFL has now made strides to address the issue by developing a technique that can perform both 3-D super-resolution microscopy and fast 3-D phase imaging in a single instrument. Phase imaging is a technique that translates the changes in the phase of light caused by cells and their organelles into refractive index maps of the cells themselves.
The unique platform, which is referred as a 4-D microscope, combines the sensitivity and high time-resolution of phase imaging with the specificity and high spatial resolution of fluorescence microscopy. The researchers developed a novel algorithm that can recover the phase information from a stack of bright-field images taken by a classical microscope.
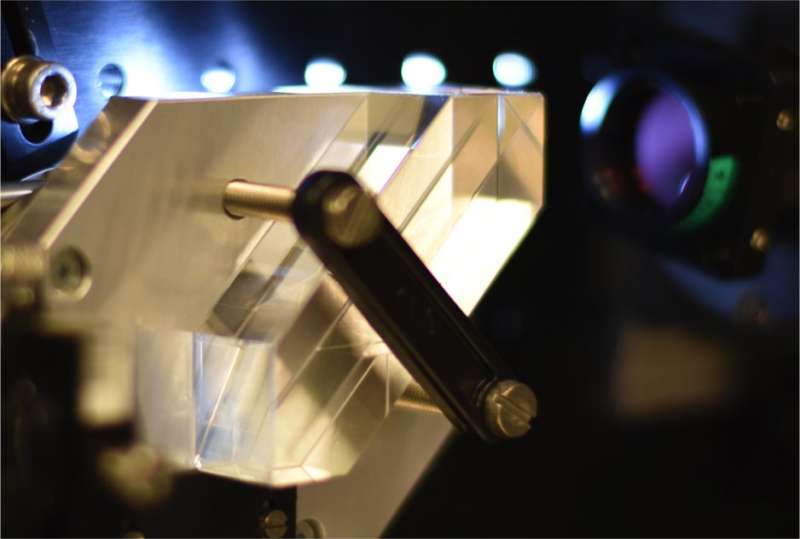
"With this algorithm, we present a new way to achieve 3-D quantitative phase microscopy using a conventional bright-field microscope," says Adrien Descloux, one of the lead authors of the paper. "This allows direct visualization and analysis of subcellular structures in living cells without labeling."
To achieve fast 3-D imaging, the scientists custom-designed an image-splitting prism, which allows the simultaneous recording of a stack of eight z-displaced images. This means that the microscope can perform high-speed 3-D phase imaging across a volume of 2.5μm x 50μm x 50μm. The microscope's speed is basically limited by the speed of its camera; for this demonstration, the team was able to image intracellular dynamics at up to 200 Hz. "With the prism as an add-on, you can turn a classical microscope into an ultra-fast 3-D imager," says Kristin Grussmayer, another one of the paper's lead authors.
The prism is also suited for 3-D fluorescence imaging, which the scientists tested using super-resolution optical fluctuation imaging (SOFI). This method exploits the blinking of fluorescent dyes to improve 3-D resolution through correlation analysis of the signal. Using this, the researchers performed 3-D super-resolution imaging of stained structures in the cells, and combined it with 3-D label-free phase imaging. The two techniques complemented each other very well, revealing fascinating images of the inner architecture, cytoskeleton, and organelles also in living cells across different time points.
"We are thrilled by these results and the possibilities offered by this technique," says Professor Hilal Lashuel, whose lab at EPFL teamed up with Professor Lasser's in using the new technique to study the mechanisms by which protein aggregation contributes to the development and progression of neurodegenerative diseases, such as Parkinson's and Alzheimer's. "The technical advances enabled high-resolution visualization of the formation of pathological alpha synuclein aggregates in hippocampal neurons."
The team has named the new microscopy platform PRISM, for Phase Retrieval Instrument with Super-resolution Microscopy. "We offer PRISM as a new microscopy tool and anticipate that it will be rapidly used in the life science community to expand the scope for 3-D high-speed imaging for biological investigations," says Theo Lasser. "We hope that it will become a regular workhorse for neuroscience and biology."
More information: A. Descloux et al. Combined multi-plane phase retrieval and super-resolution optical fluctuation imaging for 4D cell microscopy, Nature Photonics (2018). DOI: 10.1038/s41566-018-0109-4
Journal information: Nature Photonics
Provided by Ecole Polytechnique Federale de Lausanne