Intoxicatingly light-sensitive
ETH chemists have synthesised several variants of THC, the active ingredient in cannabis. Its structure can be altered with light, and the researchers have used this to create a new tool that can be used to more effectively study the body's own cannabinoid system.
When many people hear the abbreviation THC (tetrahydrocannabinol), they immediately think of smoking marijuana and intoxication. But the substance is also of interest to medicine—it relieves muscle cramps, pain, loss of appetite and nausea. THC works by binding to the corresponding cannabinoid-1 (CB1) receptors, which are located in the cell membrane and are present in large numbers in the central and peripheral nervous system. CB1 receptors play a major role in memory, motor coordination, mood and cognitive processes.
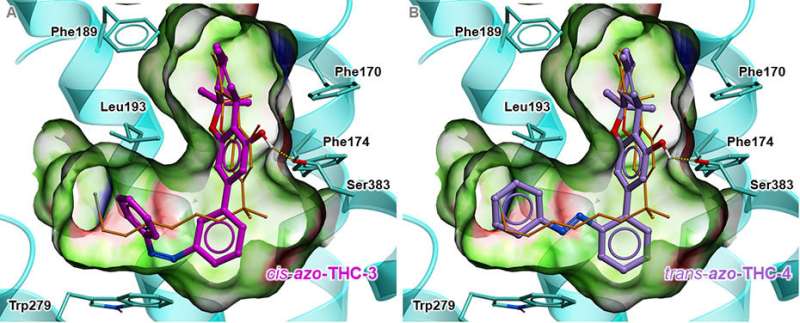
When a THC molecule binds to one of these CB1 receptors, it changes form, triggering a cascade of signals inside the cell. However, it is still hard to study CB1 receptors and their manifold functions, because cannabinoids such as THC are highly lipophilic, so they frequently embed themselves in the membranes made of fat molecules in an uncontrolled manner. To be able to use THC or its variants more precisely for pharmaceutical and medical applications, it is therefore important to gain a better understanding of CB1 receptors.
To study the diverse interactions between CB1 receptors and cannabinoids, a group of chemists headed by ETH professor Erick Carreira synthesised THC molecules. Their structure can be altered with light. The researchers published their findings in the latest issue of the Journal of the American Chemical Society.
The scientists synthesised four variants, or derivatives, of THC by attaching a light-sensitive "antenna" to the THC molecule. This antenna makes it possible to use light of a specific wavelength to precisely manipulate the altered molecule. Ultraviolet light changes the spatial structure of the antenna, and this change can be reversed again with blue light.
The researchers tested two of these derivatives in a living cell culture. The derivatives docked with CB1 receptors in the same way as naturally occurring THC. When the researchers irradiated the THC derivative with ultraviolet light, its structure altered just as the researchers expected, consequently activating the CB1 receptor. This triggers reactions such as the opening of the potassium ion channels located in the cell membrane, which causes potassium ions to flow out of the cell. The researchers were able to measure this with an electrode inserted into the cell.
When irradiated with blue light, the THC derivative returned to its original form, disabling the CB1 receptor as a result. The ion channels closed and the flow of potassium stopped. The researchers were able to activate and deactivate these processes using the corresponding coloured pulses of light.
"This work is our successful proof of principle: light-sensitive THC variants are a suitable tool for controlling and influencing CB1 receptors," says Michael Schafroth, a doctoral student with ETH professor Carreira and major contributor to the study. He added that they have now laid an important foundation for further projects that are already in progress; for example, another doctoral student in Carreira's group, Roman Sarott, is working on synthesising additional THC derivatives that react to long-wavelength red light. "Red light penetrates deeper into tissue than blue light," says Sarott. "If we want to study CB1 receptors in a living organism, we need molecules that are sensitive to red light."
In addition to the researchers from Carreira's group, leading scientists from New York University (NYU), the Indiana University Bloomington (IUB) and the University of Southern California (USC) as well as the Ludwig-Maximilian University in Munich were involved in the interdisciplinary project. The biological experiments were conducted by James Frank and Dirk Trauner.
Many cultures have long known of the intoxicating and therapeutic effect of THC. The identification of THC eventually led to the discovery of the endocannabinoid system, which involves the body's native as well as exogenous substances in the cannabinoids class as well as their receptors in the body.
The pharmaceutical industry is also interested in gaining a better understanding of the endocannabinoid system so that it can better use specific components for pharmaceutical purposes. The system is considered a possible starting point for treatments for addiction, obesity, depression and even Alzheimer's and Parkinson's.
More information: Matthias V. Westphal et al, Synthesis of Photoswitchable Δ9-Tetrahydrocannabinol Derivatives Enables Optical Control of Cannabinoid Receptor 1 Signaling, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b06456
Journal information: Journal of the American Chemical Society
Provided by ETH Zurich