Researchers identify a new chromatin regulatory mechanism linked to SirT6

Researchers from the Epigenetics and Cancer Biology Program of the Bellvitge Biomedical Research Institute (IDIBELL), led by Dr. Àlex Vaquero, have proposed a new regulation mechanism of the NF-κB pathway, which is associated with accelerated cellular aging, based on the analysis of the function of the SirT6 protein. The results of their work, published in Nature Communications, indicate a double mechanism of inhibition of the pathway linked to the action of SirT6 on chromatin.
SirT6 is a sirtuin, an enzyme involved in the cellular response to stress. Specifically, SirT6 protects the stability of the genome and regulates the metabolic balance (homeostasis), and previous studies have shown that reduction results in an accelerated aging phenotype associated with the hyperactivation of the NF-κB pathway, a family of transcription factors that regulate the cellular response to a wide variety of physiological conditions and that play a key role in cancer, inflammatory response and the immune system.
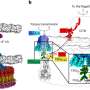
Until now, several hypotheses suggested that SirT6 kept the activation level of the NF-κB pathway low, specifically blocking it once it had received an activation signal. "In our study, however, we have identified a new mechanism associated with this regulation, whereby SirT6 not only controls NF-κB at the level of deactivation of specific repressor genes, but also controls the activation of a general repressor of the pathway," explains Dr. Àlex Vaquero, lead author of the study.
Thanks to several biochemical studies carried out in collaboration with the proteomics scientific-technical support unit of IDIBELL, researchers observed how SirT6 interacts with Suv39h1, an enzyme capable of adding methyl groups in a certain region of a histone (H3K9me3). SirT6 promotes the monoubiquitinization of Suv39h1 (Suv39h1mUb) catalyzed by the enzyme SKP2. This modification is of special interest, since it occurs in cysteine residues instead of lysine ones, something only previously observed in very few cases, and mostly in viral or peroxisome proteins. This is the first description of this modification in a nuclear factor in higher eukaryotes.
"The main function of ubiquitinization is to mark proteins by adding many units of ubiquitin for their degradation by the proteasome." In the published study, however, monoubiquitinization plays another role, since it interferes with the binding of Suv39h1 to chromatin and causes this enzyme to exit the chromatin, activating the gene that acts as a general repressor of the NF-κB pathway," adds Dr. Vaquero.
Researchers have determined that this new SirT6-dependent mechanism of inactivation of the NF-κB pathway is largely related to cellular stress levels, but there are still unresolved questions. "This work opens a new path in the study of the functions of the ubiquitination in nuclear proteins, and suggests a regulatory landscape of these modifications much more complex than previously anticipated," he says.
More information: Irene Santos-Barriopedro et al, SIRT6-dependent cysteine monoubiquitination in the PRE-SET domain of Suv39h1 regulates the NF-κB pathway, Nature Communications (2018). DOI: 10.1038/s41467-017-02586-x
Journal information: Nature Communications
Provided by IDIBELL-Bellvitge Biomedical Research Institute