Small molecules fighting aging-related diseases
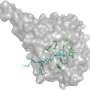
![One of the activating molecules synthesized by the research group binds to Sirtuin 6. The chemical name of the activator is “4-(pyridine-3-yl)-4,5-dihydropyrrolo[1,2-α]quinoxaline”. Credit: Clemens Steegborn Small molecules fighting aging-related diseases](https://scx1.b-cdn.net/csz/news/800a/2017/smallmolecul.jpg)
For the first time an international research network led by Bayreuth Biochemist Prof. Dr. Clemens Steegborn has succeeded in producing small molecules able to activate the enzyme sirtuin 6. Furthermore, the scientists were able to reveal the structural basis of such processes. These findings will enable the development of drugs that might support the fight against aging-related diseases.
Sirtuins are enzymes that fulfil a variety of regulatory functions in the body. In particular, they regulate energy metabolism and stress responses. The human body contains seven different sirtuins known as Sirt1 to Sirt7. The ability to selectively activate these enzymes could help in preventing or treating aging-related diseases such as certain types of cancer.
Up to now only activators for Sirt1 were known. In close collaboration with scientists at Martin Luther University Halle-Wittenberg, Sapienza University of Rome (Italy), and Stanford University (CA, USA), the research group led by Prof. Clemens Steegborn at University of Bayreuth has now succeeded in developing selective Sirt6 activators as well. These small molecules dock specifically on Sirt6, thereby increasing the activity of this enzyme. It catalyzes the removal of acetyl groups, for example from nucleosomes, which modulate the activity of genes.
Structural preconditions for targeted substances
The scientists were able to elucidate where exactly these synthetic small molecules bind to Sirt6. "Sirt6 has a unique channel, which leads from the surface of the enzyme to its catalytic center and features a binding pocket that is easily accessible from the outside. Thereby, all structural requirements are fulfilled for efficient docking of the small activators into this pocket. With a few exceptions, these small molecule activators are unable to attach to other Sirtuins, which means they are specific for Sirt6," Prof. Steegborn explains. "Our new insights offer a very promising starting point for the development of targeted compounds. These would enable further biomedical research, and they might also support therapeutic measures, e.g. in the fight against tumors," he says.
Varying effects of the substances
The activating molecules for Sirt6 are compounds based on pyrrolo[1,2-α]quinoxaline. A total of 14 compound variations were used by the researchers in Bayreuth, Halle, Rome, and Stanford in their experiments. The molecules displayed considerably differing effects on Sirt6. Some substances activated Sirt6-dependent removal of acetyl groups; other substances were limited in their effect to suppressing a different activity of the enzyme. Based on these discoveries one can imagine that one day it will not only be possible to activate Sirt6 in a targeted way, but even to achieve a more precise "fine-tuning" of the metabolic processes it regulates. "The compounds we developed and the new findings on their interaction with Sirt6 provide a unique basis for the rational design of further refined molecules. Now that we have succeeded in revealing the structural details of this interaction, we are finally in a position to modulate the enzyme sirtuin 6 very specifically with drugs," Prof. Steegborn explained.
More information: Weijie You et al. Structural Basis of Sirtuin 6 Activation by Synthetic Small Molecules, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201610082
Journal information: Angewandte Chemie International Edition
Provided by University of Bayreuth