Cells sense and explore their environments
The process through which cells are able to sense their environment is regulated by force detection. This is the main conclusion of a study published in the journal Nature, led by the team of Pere Roca-Cusachs, lecturer from the Department of Biomedicine and main researcher at the Institute for Bioengineering of Catalonia (IBEC).
"In this research, we determined how cells detect the position of molecules (or ligands) in their surroundings with nanometre precision," says Roca-Cusachs. "When the ligands join, cells apply a force they can detect. Since this force depends on the ligand spatial distribution, this enables cells to sense their surroundings. This would be the equivalent to recognizing someone's face in the dark by touching their face with your hands."
Interaction between cells and their ligands (the cell microenvironment) is essential to maintaining tissue function, and the detection of changes in the cell environment is essential in all situations where there is tissue remodelling, such as embryonic development, tumoral proliferation or the healing of a wound.
"Depending on this cell force distribution, it can affect the activation of genetic transcription, a phenomenon that determines which genes are expressed," says Roger Oria, first author of the study and Ph.D. student at the UB in Dr Roca-Cusachs' laboratory.
With this deeper knowledge of how cells detect their surroundings, researchers proved that by altering the conditions of the cell's environment (rigidity and distribution of those ligands that create the extracellular matrix), they can control the adherence response of the cell, and even define a range in which the cell adheres. This result, says Roca-Cusachs, could be important in tumoral processes, since greater rigidity is related to a higher activation of oncogenes.
Researchers have known cells are able to perceive spatial and physical information at the nanoscale. In fact, it was thought they were able to measure distances, and therefore people had hypothesized about the existence of some pattern molecule that could contribute to this process. According to the IBEC-UB researcher, this study opposes this hypothesis, since it shows that cells sense their surroundings more rather than seeing them.
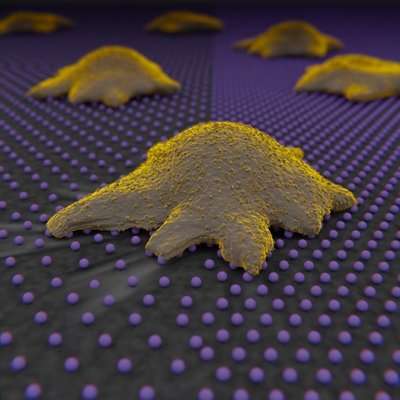
The researchers developed low-rigidity gel substrates to which a pattern of gold nanospheres covered by a protein were adhered, which controlled its separation. The cell recognizes these nanospheres as a ligand, so researchers can measure how cells regulate force distribution and the number of ligands to which they adhere regarding their density.
More information: Roger Oria et al. Force loading explains spatial sensing of ligands by cells, Nature (2017). DOI: 10.1038/nature24662
Journal information: Nature
Provided by University of Barcelona