December 4, 2017 report
Calcium compound breaks 'like repels like' rule
(Phys.org)—A combined team of chemists from the University of Bath in the U.K. and Université Toulouse III–Paul Sabatier, UMR in France has found an instance of a calcium compound breaking the 'like repels like' chemistry rule. In their paper published in the journal Science, the group describes how they accidentally came upon the reaction while studying reactions between calcium hydride and terminal alkenes. Robert Emmet Mulvey with the University of Strathclyde in the U.K. offers a Perspective piece on the work done by the team in the same journal issue.
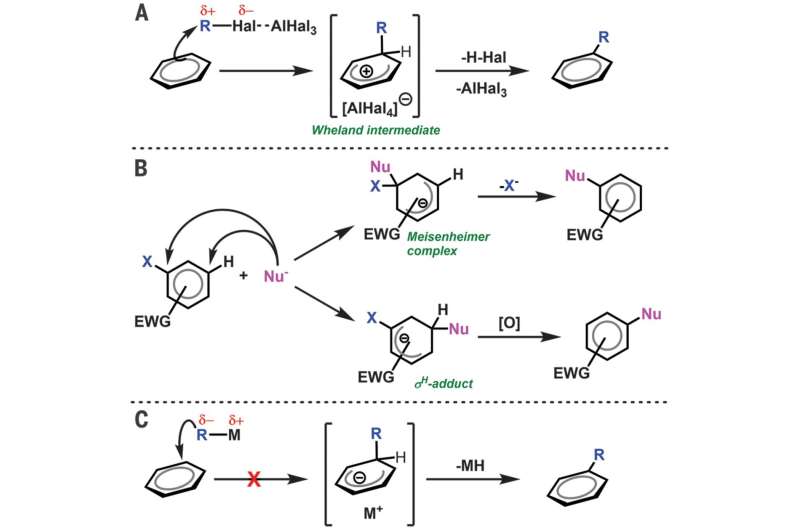
In chemistry, there is a "like repels like" rule regarding molecules such as benzene—because they are rich in electrons, it is difficult to cause them to react with nucleophiles, types of chemicals that offer up a pair of electrons to electrophiles resulting in a chemical bond. In this new effort, the researchers found an exception to this rule.
As the researchers describe it, they were studying reactions between terminal alkenes and a calcium hydride and became impatient waiting for a reaction to occur. In an effort to speed things up, they applied some heat—doing so, they discovered, resulted in a reaction that produced alkylbenzenes, something that was unexpected. Suspecting that it might be a nucleophilic attack led the team to investigate further. They found that the calcium set up the benzene for the suspected nucleophilic attack and also pulled electron density away from the aromatic ring. That was followed by the alkyl substituent moving to the benzene and the removal of the hydrogen atom, with the end result being the production of a benzene that had a linear alkane chain. The "like repels like" rule had been broken, the group notes, by the demonstration of a calcium compound allowing an electron-rich benzene to react with an electron-rich reagent.
The researchers acknowledge that they can think of no useful purpose for what they discovered, but suggest their findings might lead to discovering other surprising reactions, broadening the science. It might also serve as a reminder for other chemists conducting research to remain open-minded about established rules that might sometimes be broken under certain conditions.
More information: Andrew S. S. Wilson et al. Organocalcium-mediated nucleophilic alkylation of benzene, Science (2017). DOI: 10.1126/science.aao5923
Abstract
The electrophilic aromatic substitution of a C–H bond of benzene is one of the archetypal transformations of organic chemistry. In contrast, the electron-rich π-system of benzene is highly resistant to reactions with electron-rich and negatively charged organic nucleophiles. Here, we report that this previously insurmountable electronic repulsion may be overcome through the use of sufficiently potent organocalcium nucleophiles. Calcium n-alkyl derivatives—synthesized by reaction of ethene, but-1-ene, and hex-1-ene with a dimeric calcium hydride—react with protio and deutero benzene at 60°C through nucleophilic substitution of an aromatic C–D/H bond. These reactions produce the n-alkyl benzenes with regeneration of the calcium hydride. Density functional theory calculations implicate an unstabilized Meisenheimer complex in the C–H activation transition state.
Journal information: Science
© 2017 Phys.org