Superconductivity-like electron pair formation in molecules discovered
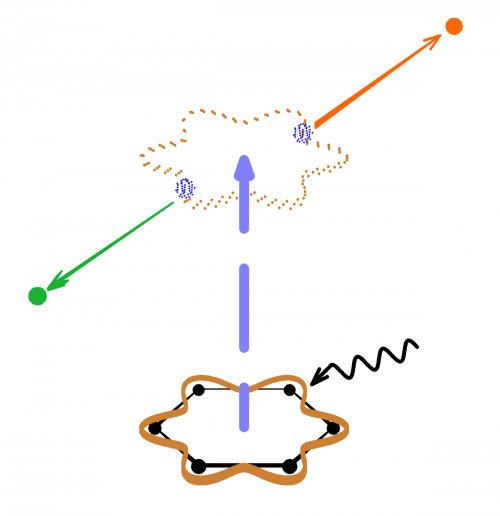
(Phys.org)—Usually, electrons try to avoid each other due to their electrostatic repulsion. On occasion, however, they can form a pair which has long been known in superconductivity called a "Cooper pair," named after the physicist who first described them. In work published in Physical Review Letters, researchers from the Wehlitz group at SRC outline their discovery that electrons can form pairs in some aromatic molecules as small as benzene. A benzene molecule consists of only 6 carbon atoms in a ring with one hydrogen atom attached to each of them.
The benzene ring allows the electrons, when the molecule absorbs a photon with a certain amount of energy, to flow around in a ring-shaped orbital where they can form a 2-electron pseudo particle just like a Cooper pair. This pair formation is, on the one hand, independent of the specific molecule (i.e. is the same for benzene naphthalene, anthracene) but, on the other hand, does not exist at all for other molecules like pyrrole (a pentagonal ring with four carbon and one nitrogen atom).
This finding opens a new avenue in the quest for understanding high temperature superconductors and, ultimately, in the search for room temperature superconductors. While benzene is not a superconductor, the data shows the formation of a Cooper pair, which is a requirement for superconductivity. Thus benzene can be used as a convenient model system to study Cooper pair formation.
More information:
PRL paper: prl.aps.org/abstract/PRL/v109/i19/e193001
Synopsis: physics.aps.org/synopsis-for/1 … ysRevLett.109.193001
Journal information: Physical Review Letters
Provided by University of Wisconsin-Madison