November 21, 2017 report
Using magnets to control chemical reactions that target release of medicines inside the body
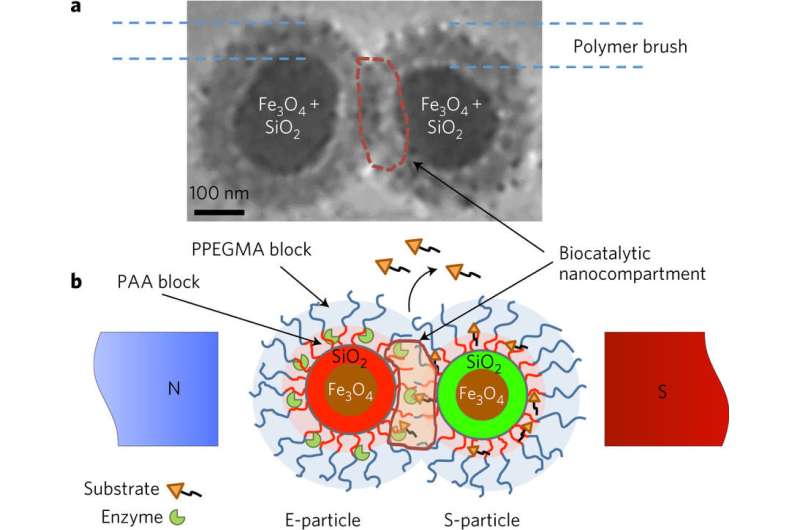
(Phys.org)—A team of researchers with the University of Georgia in Athens has developed a technique for controlling chemical reactions that release drugs inside the body. In their paper published in the journal Nature Catalysis, the group describes coating chemicals to prevent a reaction from occurring until the application of a magnetic field that releases a desired drug.
In some medical applications, it is better for a medical treatment if a chemical can be applied directly to a certain part of the body and nowhere else. Chemicals meant to treat tumors are the prime example—chemotherapy drugs act on every cell they contact, causing a host of negative side effects. In this new effort, the group took a novel approach to solving this problem, using a magnet to force coated chemicals together, prompting a drug releasing reaction.
To provide a means for controlling when chemicals come into contact inside the body, the researchers created tiny packets by first coating iron oxide nanoparticles with silica and then coating them further with two types of polymers, which, when combined, form a brush-like structure. Each of the packets was then loaded with either an enzyme or a substrate meant to react with the enzyme, and, of course, the drug to be released.
In practice, the packets would be released into the body of a patient, where they would make their way to the whole body, behaving harmlessly, as the brushes prevent them from reacting whenever they meet. When the packets made their way to a site where a reaction was wanted, the researcher applied a magnet that forced them close together—close enough that they could react, releasing the drug. The other packets not involved in the reaction would slowly be flushed out of the body naturally, without causing harm.
The researchers tested their packets in vitro using a real chemotherapy drug and cancer cells. They report it worked just as they had envisioned. More testing is required, of course, to ensure the technique is safe, but if all goes well, it could eventually be used to treat a wide variety of cancers.
More information: Andrey Zakharchenko et al. Magnetic field remotely controlled selective biocatalysis, Nature Catalysis (2017). DOI: 10.1038/s41929-017-0003-3
Abstract
Many applications for medical therapy, biotechnology and biosensors rely on efficient delivery and release of active substances. Here, we demonstrate a platform that explores magnetic-field-responsive compartmentalization of biocatalytic reactions for well-controlled release of chemicals or biological materials on demand. This platform combines two different kinds of core–shell magnetic nanoparticle: one loaded with enzymes and another with substrate-bound therapeutic (bio)chemicals. Both cargos are shielded with a polymer brush structure of the nanoparticle shell, which prevents any enzyme–substrate interactions. The shield's barrier is overcome when a relatively weak (a fraction of 1 T) external magnetic field is applied and the enzyme and the substrate are merged and forced to interact in the generated nanocompartment. The merged biocatalytic nanoparticles liberate the substrate-bound therapeutic drugs when the enzymes degrade the substrate. The developed platform provides a proof of concept for the remotely controlled release of drugs or (bio)chemicals using the energy of a non-invasive, weak magnetic field.
Journal information: Nature Catalysis
© 2017 Phys.org




















