Researchers inadvertently boost surface area of nickel nanoparticles for catalysis
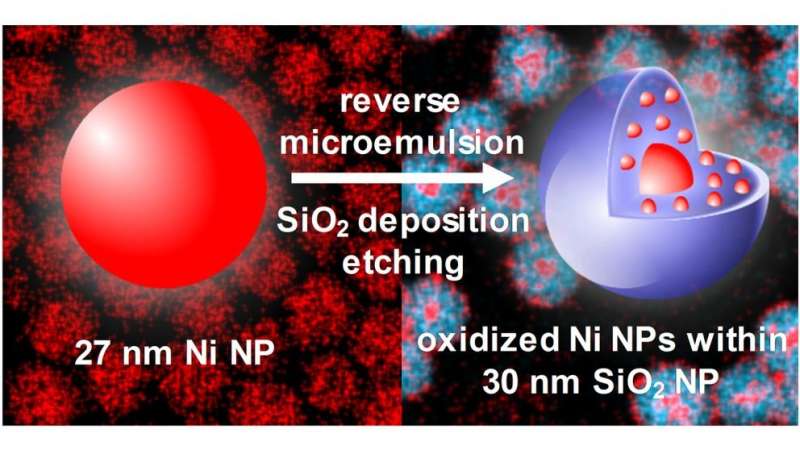
Researchers from North Carolina State University and the Air Force Research Laboratory have discovered that a technique designed to coat nickel nanoparticles with silica shells actually fragments the material – creating a small core of oxidized nickel surrounded by smaller satellites embedded in a silica shell. The surprising result may prove useful by increasing the surface area of nickel available for catalyzing chemical reactions.
"Nickel is noteworthy for its widespread applications in catalysis," says Joe Tracy, an associate professor of materials science and engineering at NC State and corresponding author of a paper on the work. "One reason you'd want to coat nickel nanoparticles in porous silica is to embed them in a neutral substrate to maintain their efficiency as catalysts in chemical reactions. So the fact that this process could increase their surface area at the same time could prove to be beneficial."
The researchers employed a widely used approach called reverse microemulsion, or reverse micelle, to apply a silica coating to nickel nanoparticles that were approximately 27 nanometers (nm) in diameter. But they found that the technique results in an oxidized nickel core that was 7 nm in diameter, surrounded by oxidized nickel satellites only 2 nm in diameter – all enclosed in a silica shell that was 30 nm in diameter.
"At first we thought we'd made a mistake, but we were able to reproduce the result over and over again," says Brian Lynch, a Ph.D. student at NC State and lead author of a paper on the work.
"When oxidized and reduced at high temperatures, we found that the core-and-satellite nickel nanoparticles did not significantly change size or shape, suggesting that they would function well in the environments needed to catalyze chemical reactions," Tracy says.
"This was an unexpected discovery, but we're happy with how it turned out."
The paper, "Synthesis and Chemical Transformation of Ni Nanoparticles Embedded in Silica," is published in the journal Nanoscale.
More information: Brian B. Lynch et al. Synthesis and chemical transformation of Ni nanoparticles embedded in silica, Nanoscale (2017). DOI: 10.1039/C7NR06379B
Journal information: Nanoscale