Researchers mimic two natural energy processes with a single catalyst
Nature is quite good at doing certain kinds of chemistry. For example, water is continuously transformed into its constituents, oxygen, protons, and electrons, and back again as a way of storing and using energy by plants and animals. Technologies based on natural chemical pathways could help to meet mankind's growing energy demands. Specialized enzymes present in plant and animal cells for certain chemical reactions have inspired chemists to try and reproduce natural processes in artificial solar cells and fuel cells.
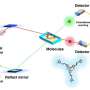
Now, researchers based at Kyushu University have developed a single catalyst capable of acting as both a fuel cell that consumes hydrogen to release energy and a photosynthetic system able to make oxygen using solar energy. The group recently reported their findings in ChemCatChem.
"People have tried before to artificially replicate the behavior of hydrogenase and photosystem II," says corresponding author Professor Seiji Ogo of Kyushu University."But ours is the first study to combine these two very specific biological functions into a single catalytic system that can do both."
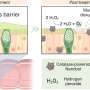
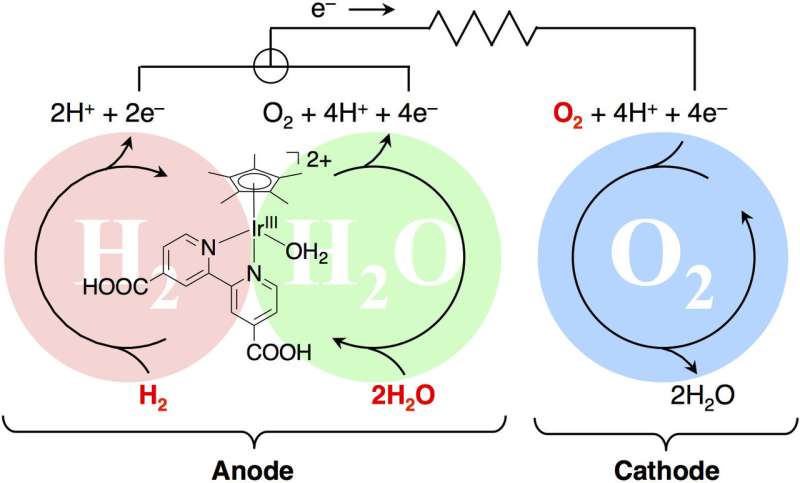
Hydrogenase is an enzyme present in organisms that acts like a natural fuel cell, consuming hydrogen for energy. Photosystem II allows plants to turn water into oxygen under sunlight. Both these processes involve oxidations, where either hydrogen or water molecules give up some of their electrons.
The researchers synthesized a catalyst containing the metal iridium, which is capable of accepting and releasing a number of electrons. They showed that in a fuel cell, their catalyst produced electrical power by accepting electrons from hydrogen. Changing the supporting materials in the catalyst could generate power from sunlight through a cycle involving oxidation of water.
The researchers managed to isolate the chemical and use X-ray diffraction to provide new insights into its structure and behavior as a catalyst for the first time. Corresponding author Professor Seiji Ogo says, "The power output of our system is still rather low for any practical applications, but this work represents a unique demonstration of two different kinds of energy generating processes from a single catalyst. We hope these findings will show that chemists still have much to learn from natural processes."
More information: Mitsuhiro Kikkawa et al, A Fusion of Biomimetic Fuel and Solar Cells Based on Hydrogenase, Photosystem II, and Cytochrome c Oxidase, ChemCatChem (2017). DOI: 10.1002/cctc.201700995
Provided by Kyushu University