High-resolution structural analysis of protein behind Huntington's disease
Huntington's disease is a progressive, fatal neurodegenerative disorder that is caused by mutations in one specific gene called huntingtin (Htt). In the 20-plus years since the Htt gene was identified, researchers have focused on the protein encoded by the Htt gene, called Httex1. This protein accumulates in the brains of Huntington's disease patients, and the prevailing hypothesis has been that it undergoes a dramatic structural change when a repetitive tract of the amino acid glutamine mutates into an aberrantly long region known as the mutationally expanded polyglutamine (polyQ) tract.
Now, for the first time, the team of Hilal A. Lashuel at Ècole Polytechnique Fèdèrale de Lausanne (EPFL) in Switzerland; Edward A. Lemke at the European Molecular Biology Laboratory (EMBL) in Germany; and Rohit V. Pappu at Washington University in St. Louis has uncovered a detailed structural description of Htt as a function of polyQ length. The work was published recently in the Journal of the American Chemical Society.
A study in three steps
Securing atomic-level structural descriptions of full-length Htt and disease-relevant protein fragments referred to as Httex1 have been challenging because these molecules stick to one another and inhibit the generation of pure protein samples for structural studies. "It is very difficult to obtain structural characterization of proteins within a mush," said Pappu, the Edwin H. Murty Professsor of Engineering in the School of Engineering & Applied Science.
"Our goal was to gain insight into how increasing the length of the polyQ tail repeat alters structure of this protein at the monomer level and under conditions where we are able to unlink its folding and self-assembly," said Lashuel, professor of life sciences and director of the laboratory of the chemical biology of neurodegeneration at EPFL.
In the first step of the study, Lashuel and postdoctoral fellow John B. Warner IV used novel chemical strategies in their lab to produce precise, high-purity samples of Htt for molecular spectroscopy. But these only came in ultra-low concentrations and required techniques that probe individual molecules. Warner and Lashuel enabled these experiments by generating samples with site-specific fluorescent labels.
For the second step of the project, Warner and Lashuel worked with Lemke's lab at EMBL to perform single-molecule Förster (or fluorescence) resonance energy transfer (smFRET), which is a technique that can measure distances between 1-10 nanometers within individual molecules—in this case, within individual Htt proteins. This part of the study yielded the first quantitative assessment of how the inter-atomic distances within Httex1 vary with the expansion mutations.
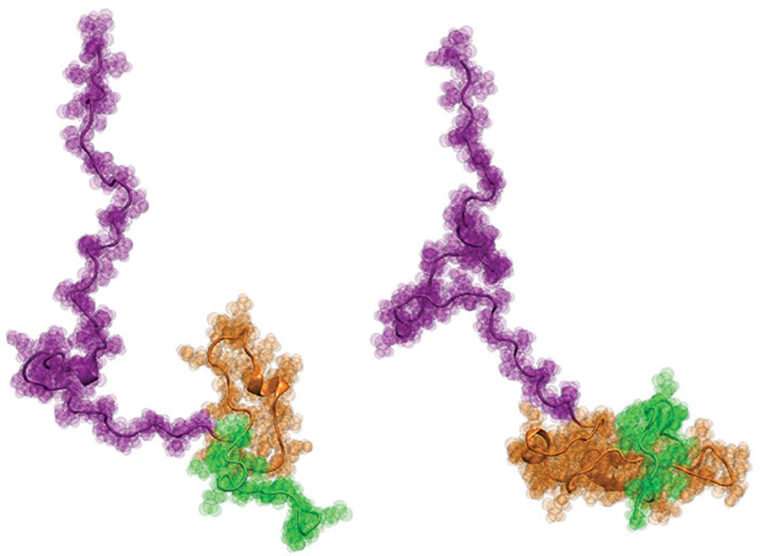
Finally, the scientists worked with Pappu's lab at Washington University, where it developed novel computer modeling approaches to produce physically accurate, atomic-level structural models of Httex1 that best fit all of the single-molecule data from the previous two steps. The results were surprising: The overall structure of Httex1 resembles that of a tadpole.
The tadpole effect
"Architecturally, Httex1 is tadpole-shaped, with a globular polyQ head and a floppy tail," Pappu said. "As the polyQ length gets longer, the head of the tadpole becomes larger in its surface area. This increased surface area of the head appears to engender interactions that otherwise shouldn't be present in cells."
The discovery challenges the longstanding ideas about Httex1 accumulation in Huntington's disease. "If the prevailing hypothesis were true," Pappu said, "then the tadpole would have turned into a 'frog' as the polyQ length increases above the threshold length, but that does not appear to be the case. The new results instead focus our attention on the novel gain-of-function cellular interactions that are driven by the tadpole structure with a larger polyQ head."
"While the prevailing hypothesis has favored a model where mutant huntingtin-induced toxicity is driven mainly by its propensity to misfold and aggregate, our findings suggest that aberrant interactions at the monomer level may also contribute to the initiation and/or progression of the disease," Lashuel said.
"This finding allows us to examine what regions of this protein are important to target, and modulate its toxicity in a specific manner," said Kiersten M. Ruff, a postdoctoral fellow in Pappu's lab who designed the computer simulations and is the co-first author on the paper.
The next challenge for the scientists is to understand how these structural changes at the monomer level of Httex1 translate into increased aggregation and toxicity when the length of the polyQ tail crosses the pathogenic threshold.
"The key has been the centrality of collaboration among three teams with complementary and non-overlapping expertise, all sharing a commitment to advancing science," Lashuel said.
More information: John B. Warner et al. Monomeric Huntingtin Exon 1 Has Similar Overall Structural Features for Wild-Type and Pathological Polyglutamine Lengths, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b06659
Journal information: Journal of the American Chemical Society
Provided by Washington University in St. Louis