Model predicts how E. coli bacteria adapt under stress
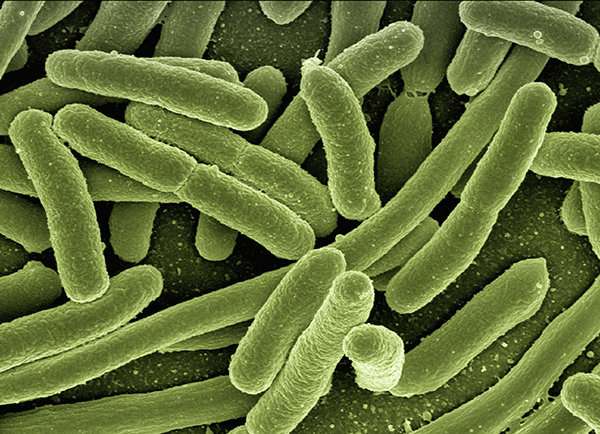
Researchers at the University of California San Diego have developed a genome-scale model that can accurately predict how E. coli bacteria respond to temperature changes and genetic mutations. The work is aimed at providing a comprehensive, systems-level understanding of how cells adapt under environmental stress. The work has applications in precision medicine, where adaptive cell modeling could provide patient-specific treatments for bacterial infections.
A team led by Bernhard Palsson, a professor of bioengineering at UC San Diego, published the work on Oct. 10 in Proceedings of the National Academy of Sciences.
"In order to have full control over living cells, we need to understand the fundamental mechanisms by which they survive and quickly adapt to changing environments," said Ke Chen, a postdoctoral researcher at UC San Diego and the study's first author.
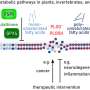
A fundamental principle behind this work is that changes in the environment cause changes in a cell's protein structure. For example, higher temperatures destabilize protein molecules. The new genome-scale computational model, called FoldME, predicts how E. coli cells respond to temperature stress and then reallocate their resources to stabilize proteins. "The more the proteins destabilize, the more resources are devoted to re-stabilize them, making resources less available for growth and other cellular functions," Palsson explained.
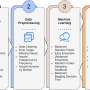
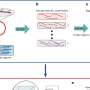
To construct FoldME, the team first compiled the structures of all the protein molecules in E. coli cells and then integrated that data into existing genome-scale models of metabolism and protein expression for E. coli. Next, they calculated a biophysical profile that represents how well each protein folds at different temperatures. Since proteins usually need small molecules called chaperones to help them fold at high temperatures, the researchers also incorporated chaperone-assisted folding reactions into the model. They then set the model to maximize cell growth rate.
FoldME accurately simulated the response of E. coli cells throughout a wide temperature range and provided details on the strategies they used to adapt at each different temperature. The model's predictions were consistent with experimental findings. For example, it correctly reproduced the variations in E. coli cell growth rate at different temperatures. FoldME simulations also showed that E. coli cells consume a different type of sugar at high temperatures.
The model also evaluated how mutations in a single gene affect E. coli cells' response to stress. It predicted that point mutations in a single metabolic gene called DHFR result in the differential expression of a large number of proteins. This was also confirmed by experimental findings.
Another important aspect of this work is that it highlights the systems-level regulatory role of the chaperone network, which has been overlooked in previous studies, Chen said. Chaperones provide a critical service in that they help proteins fold under stress (at higher temperatures), but their service is a limited resource that's shared by all the proteins in the cell. Helping one protein fold means a chaperone isn't available to help other proteins to fold—a limitation that affects the structural integrity of the rest of the cell's proteins. This also drains available resources from protein synthesis, setting a stringent translational constraint on all the proteins, researchers explained.
"Using first principles calculations, we can get a deep understanding of how multiple protein folding events, chaperone regulation and other intracellular reactions all work together to enable the cell to respond to environmental and genetic stresses," Chen said.
"It is worth noting that we know that adaptation to chemical stress and changing nutrients typically only require a handful of mutations, while adaptation to temperature stress is much more difficult and predicted to require a large number of mutations," Palsson added.
Next steps involve experimental tests on the model that are aimed at exploring how bacteria adapt at higher temperatures. The team is also planning to study the adaptation processes of other disease-causing bacteria—such as diarrhea-causing E. coli, M. tuberculosis and staph bacteria—under stresses that mimic conditions in their native human habitats.
More information: Ke Chen et al, Thermosensitivity of growth is determined by chaperone-mediated proteome reallocation, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1705524114
Journal information: Proceedings of the National Academy of Sciences
Provided by University of California - San Diego