Maximum precision in protein synthesis
Researchers from the Center for Molecular Biology of Heidelberg University (ZMBH) and the German Cancer Research Center (DKFZ) have investigated the mode of action of a molecular chaperone vital to protein synthesis. Together with colleagues from the University of Cologne and the Penn State University (USA), they were able to demonstrate that the speed of protein synthesis is associated with the function of the Ssb chaperone. The information controlling synthesis speed is stored in the genetic code of the cell, thus ensuring maximum efficiency and precision in synthesising functional proteins. The results of their research were published in Cell.
Every cell contains thousands of different proteins, each with a specific function in the organism. The blueprints for their synthesis are stored in the genes. Large molecular machines in the cell, the ribosomes, read the information and use it to synthesise long amino acid chains. These so-called polypeptides then need to "fold" into three-dimensional structures to create functional proteins. Protein synthesis is highly complex and prone to error, so molecular folding helpers known as chaperones surveil and guide this vital process.
Some chaperones bind directly to ribosomes to help with folding during the synthesis of polypeptides. Until now, virtually nothing was known about how these ribosome-associated chaperones identify the growing proteins and help with the folding process, exactly which proteins come in contact with the chaperone, and whether the speed of protein synthesis is adapted to the function of the folding helper.
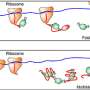
The German-American team led by ZMBH and DKFZ researchers Prof. Dr Bernd Bukau and Dr Günter Kramer delved into these questions using the yeast Hsp70 heat shock proteins as a model. Their research focused on the chaperone Ssb, the only member of the ubiquitous Hsp family that comes into direct contact with the ribosome and is involved in protein synthesis very early on in the process. In their experiments, the researchers gained fundamentally new insights into the folding of newly synthesised proteins.
The ribosome-associated Ssb Hsp70 chaperone binds to approximately 70 percent of newly forming cellular proteins, including many that must first be transported to another area of the cell before folding. In addition to its function as a folding helper, Ssb has an additional and unexpected role in cellular protein transport.
The chaperone recognises its growing protein substrates by binding a sequence stretch enriched with positively charged and nonpolar amino acids. These recognition motifs are bound as soon as they appear on the surface of the ribosome after synthesis. Ssb thereby delays the folding process until the polypeptide is long enough to fold in the correct way.
Researchers were surprised to discover that the ribosomes increase the speed of protein synthesis when the Ssb Hsp70 chaperone binds and protects the emerging polypeptide. They demonstrated that the information to adjust the speed of synthesis to the function of the molecular chaperone is stored in the genetic code and is not controlled by the chaperone itself.
More information: Kristina Döring et al. Profiling Ssb-Nascent Chain Interactions Reveals Principles of Hsp70-Assisted Folding, Cell (2017). DOI: 10.1016/j.cell.2017.06.038
Journal information: Cell
Provided by Heidelberg University