How an interest in bipolar disorder drugs led to a better understanding of leukemia
A research project that began 20 years ago with an interest in how lithium treats mood disorders has yielded insights into the progression of blood cancers such as leukemia. The research, which centers on a protein called GSK-3, will be published in the Nov. 3 issue of the Journal of Biological Chemistry.
Lithium is considered a highly effective treatment for bipolar disorder and other mood disorders, but it still works in only a fraction of patients and has a number of side effects. Furthermore, its mechanism of action is poorly understood, hampering efforts to improve on it.
In 1996, Peter Klein of the University of Pennsylvania discovered that one of lithium's biological activities was inhibiting GSK-3, an enzyme that modifies other proteins by attaching phosphate molecules, a process called phosphorylation. Lithium's effect on GSK-3 affected the development of animal cells, but it is still unknown what connection, if any, this has to psychiatric disease.
Since then, Klein - now a professor of medicine at the University of Pennsylvania - has been investigating many different aspects of GSK-3 activity. "In this paper, we were trying to find out what proteins in the cell are affected by GSK-3 inhibition," Klein said. "We compared cells with GSK-3 to cells completely lacking GSK-3 to ask how other proteins changed."
"Mood disorders are so multifaceted in terms of the pathways and pathologies involved; it's really difficult to pin down a specific pathway," said Mansi Shinde, a former graduate student in Klein's research group who led the new study. "We said: Let's look at what GSK-3 does, and that would maybe lead us toward what lithium does."
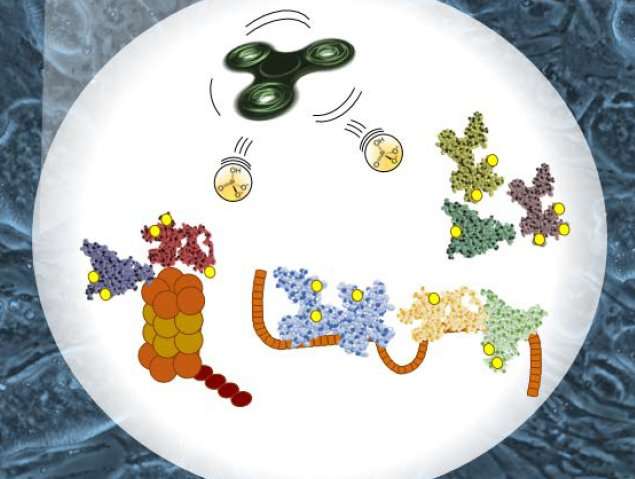
The research team used mass spectrometry to compare phosphorylation of proteins from mouse embryonic stem cells with fully functioning GSK-3 to cells in which the gene encoding GSK-3 had been deleted. The resulting massive dataset is called a phosphoproteome - a comprehensive catalog of proteins that are phosphorylated by GSK-3. Analyzing the data yielded some surprising findings.
Conventional wisdom had suggested that GSK-3 phosphorylates proteins that contain a specific amino acid sequence, but the new phosphoproteome showed that the majority of proteins whose phosphorylation depended on GSK-3 did not contain this sequence. Notably, the phosphorylated proteins included a group called splicing factors, which splice together different sections of messenger RNA, changing the proteins that they encode. Absence of GSK-3 changed the splicing patterns of more than 200 messenger RNAs.
The finding that GSK-3 could affect RNA splicing pointed to an unexpected connection: leukemia. Several factors newly discovered to be phosphorylated by GSK-3 are also known to be mutated in acute myeloid leukemia, a condition in which aberrant splicing causes uncontrolled white blood cell proliferation. This observation could also explain why one of the side effects of taking lithium is increased white blood cell count.
"The effect on the splicing factors and other mutations associated with leukemia was completely surprising to me," Klein said. The group is therefore now pursuing investigations into how GSK-3 affects the growth of healthy and leukemic blood cells.
Shinde and Klein are not yet sure whether GSK-3's effect on RNA splicing explains its role in mood disorders. The effect of GSK-3 on messenger RNA in neuronal cells, with or without lithium, would need to be examined to determine this.The study underlines how investigations into the basic biological function of a drug target can lead in unexpected directions. "[The GSK-3 phosphoproteome] is a really large data set," Shinde said. "It's a resource for the field.""The relevance to leukemia could be direct and something worthy of immediate study," Klein said. "The role in psychiatric disorders is a major interest of the work, but the impact would be down the road, not immediate."
More information: Mansi Y. Shinde et al, Phosphoproteomics reveals that glycogen synthase kinase-3 phosphorylates multiple splicing factors and is associated with alternative splicing, Journal of Biological Chemistry (2017). DOI: 10.1074/jbc.M117.813527
Journal information: Journal of Biological Chemistry