Study reveals exactly how low-cost fuel cell catalysts work
In order to reduce the cost of next-generation polymer electrolyte fuel cells for vehicles, researchers have been developing alternatives to the prohibitively expensive platinum and platinum-group metal (PGM) catalysts currently used in fuel cell electrodes. New work at Los Alamos and Oak Ridge national laboratories is resolving difficult fuel-cell performance questions, both in determining efficient new materials and understanding how they work at an atomic level. The research is described this week in the journal Science.
"What makes this exploration especially important is that it enhances our understanding of exactly why these alternative catalysts are active," said Piotr Zelenay, leader of the project at Los Alamos National Laboratory. "We've been advancing the field, but without understanding the sources of activity; without the structural and functional insights, further progress was going to be very difficult."
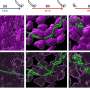
Building on previous studies, the Los Alamos-led team has synthesized catalysts comprising low-cost platinum alternatives that yield performance comparable to the standard PGM fuel cell catalyst used in vehicle applications. Using sophisticated microscopy at Oak Ridge National Laboratory (ORNL), researchers were able to directly observe the single-atom active sites in the novel material where catalysis takes place, which provided unique insights into the PGM-free material's efficiency potential.
Platinum aids in both the electrocatalytic oxidation of hydrogen fuel at the anode and electrocatalytic reduction of oxygen from air at the cathode, producing usable electricity. Finding a viable, low-cost PGM-free catalyst alternative is becoming more and more possible, but understanding exactly where and how catalysis is occurring in these new materials has been a long-standing challenge. This is true, Zelenay noted, especially in the fuel cell cathode, where a relatively slow oxygen reduction reaction, or ORR, takes place that requires significant 'loading' of platinum.
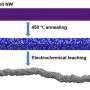
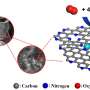
The new material examined in this study is an iron-nitrogen-carbon (Fe-N-C) electrocatalyst, synthesized with two nitrogen precursors that developed a hierarchical pore structure to expose a large fraction of the carbon surfaces to oxygen. Its fuel-cell performance is approaching that of platinum catalysts, a significant advance, as documented in fuel cell test-stand performance.
Through the use of ORNL's aberration-corrected scanning transmission electron microscope and electron energy loss spectroscopy, ORNL researchers were able to provide the first direct observation of the often proposed ORR active site, FeN4, at an atomic level.
"With both this performance and the atomic visualization of the reaction sites, we are closing the gap to replace platinum with a high-performance catalyst poised to be scaled up for potential application in fuel cells for automotive applications," said Karren More, ORNL microscopy team lead.
In addition, the high activity of Fe-N-C catalysts and the FeN4 active-site structure was predicted by computer modeling conducted at Los Alamos, as was the possible reaction pathway.
"In this paper we're tying the modeling and the microscopy results with the electrochemically determined high activity of a PGM-free oxygen reduction reaction catalyst," Zelenay said.
Los Alamos research into fuel cells expands the options for energy production in support of the Laboratory's mission of strengthening the nation's energy security.
More information: "Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst" Science (2017). science.sciencemag.org/cgi/doi … 1126/science.aan2255
Journal information: Science
Provided by Los Alamos National Laboratory