Country's largest estuary facing increasing acidification risk

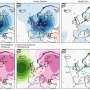
Chesapeake Bay, the largest estuary in the United States and one of the largest in the world, is facing new risks from a layer of highly acidified water some 10 to 15 meters below the surface, a new study has found.
This "pH minimum zone" is 10 times more acidic than the bay's surface waters and may pose a risk to a variety of economically and ecologically important marine species, including oysters, crabs and fish, the researchers say. A decline in the number of calcium carbonate-shelled organisms - particularly oysters - may be hampering the bay's ability to deal with the increase in acidity, they add.
Results of the study are being reported this week in Nature Communications.
"Oysters and other bivalves provide a built-in Tums effect that naturally helps the bay deal with corrosive water," said George Waldbusser, an Oregon State University marine ecologist and co-author on the study. "They generate large amounts of calcium carbonate structures, which may be able to buffer the increasing amounts of carbon dioxide entering the bay.
"Overharvesting and disease have reduced the number of oysters, however, and we're seeing the results."
Lead author Wei-Jun Cai from the University of Delaware led the study, which found pH levels in this stratified layer of water to be about 7.4, nearly a unit lower than surface waters where the average pH is about 8.2. A combination of factors likely caused this corrosive zone, including hypoxia and generation of toxic hydrogen sulfide in the bottom waters mixing with other layers of the bay.
"This study shows for the first time that the oxidation of hydrogen sulfide and ammonia from the bottom waters could be a major contributor to lower pH in coastal oceans and may lead to more rapid acidification in coastal waters compared to the open ocean," Cai said.
Previous studies, including work by Waldbusser, have shown that agricultural nutrients entering Chesapeake Bay have progressively depleted oxygen levels in the bottom waters - a process known as hypoxia - as well as acidifying the bay more quickly than offshore ocean waters. Animals need oxygen to live and without it, they die. Bacteria, however, can "breathe" without oxygen, often producing hydrogen sulfide, which further increases oxygen demand and also enhances acidification, Waldbusser said.
"Hypoxia in this case leads to an amplification of acidification," he pointed out. "If more oysters were there, they would help pull the food out of the water, reduce oxygen demand, and sequester carbon from the system. Now the acidification is such that we have to be concerned that it will make it harder for some marine organisms to produce their calcium carbonate shells. We don't yet know what those thresholds are all around."
Oysters have been shown to be sensitive to changes in acidifications, particularly on the West Coast where corrosive waters severely affected the industry several years ago. Waldbusser and OSU colleague Burke Hales helped growers mitigate the issue by identifying times of the day when local acidification levels were lower so hatcheries could draw in more favorable waters to use in raising their oysters.
East Coast oysters are a different variety, Waldbusser said, and may actually be somewhat more resilient than the West Coast's Pacific oysters. But scientific understanding of how much acidity they can withstand is limited.
"We know that in some areas of Chesapeake Bay where there has been high acidity, oysters have survived, but we don't know if there are sub-populations that have more resilience, or what the threshold is for their ability to create shells."
Waldbusser said individual oysters can filter upwards of 50 gallons of water each day. Researchers have estimated that prior to European settlement, Chesapeake Bay had so many oysters that they could filter the entire bay in three days. Today, it would take roughly 300 days because of fewer oysters and more nutrients in the water, he said.
"Dredging of the bay in the 1950s and 1960s removed a lot of oyster shells that formed a base for creating oyster reefs," said Waldbusser, who began his research on oysters and acidification at the University of Maryland more than 10 years ago before coming to Oregon State.
"Since the 1980s, many of the restaurants on the East Coast participated in a program to recycle oyster shells into the bay to create more habitat, but it hasn't been enough to replace what has been taken out."
Journal information: Nature Communications
Provided by Oregon State University