Scientists develop fast chemistry that unlocks a new class of polymers
A team of researchers has developed a faster and easier way to make sulfur-containing polymers that will lower the cost of large-scale production.
The achievement, published in Nature Chemistry and Angewandte Chemie, opens the door to creating new products from this class of polymers while producing far less hazardous waste. The researchers' reaction technique, dubbed SuFEx for sulfur(VI) fluoride exchange, combined with a newly identified class of catalysts that speed up the reactions, could be used to make everything from water bottles and mobile phone cases to medical devices and bulletproof glass.
When a useful molecule is discovered, there are few reactions that chemists can use that are simple and efficient enough to meet the industrial production requirements for cost-effectively scaling up. In 2001, Nobel laureate K. Barry Sharpless introduced a new concept to organic chemistry known as "click chemistry," describing a suite of controllable, highly reactive reactions that are high-yielding and require little to no purification.
Following nature's example, click reactions follow simple protocols, use readily available starting materials, and work under mild reaction conditions with benign starting reagents. Click chemistry has become a valuable tool for generating large libraries of potentially useful compounds as industries look to discover new drugs and materials.

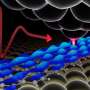
Scientists at Lawrence Berkeley National Laboratory's (Berkeley Lab) Molecular Foundry, a facility that specializes in nanoscale science, worked with a team led by Sharpless and Peng Wu, professors at the Scripps Research Institute (TSRI). The team created long chains of linked sulfur-containing molecules, termed polysulfates and polysulfonates, using a SuFEx click reaction.
"Click chemistry is a powerful tool for materials discovery, but synthetic chemists are often not well-equipped to characterize the polymers they create," said Yi Liu, director of the Organic Synthesis facility at the Molecular Foundry. "We can provide a broad spectrum of expertise and instrumentation that can expand the scope and impact of their research."
The SuFEx reaction, introduced as a new family of click reactions in 2014, reliably and quickly creates new chemical bonds, connecting compounds together with sulfates or sulfonates. While polysulfates have shown great potential as competitors to polycarbonates (strong plastics used for eyewear lenses and water bottles, for example), they have been rarely used for industrial applications due to a lack of reliable and easily scalable synthetic processes.
To overcome the challenges of mass-manufacturing polysulfates and polysulfonates, the TSRI team explored various catalysts and starting reagents to optimize the SuFEx reaction. They relied on their collaborators at the Molecular Foundry to assess physical properties and determine if the newly created polymers were thermally stable products.

Polymers are assembled from smaller molecules - like stringing a repeating pattern of beads on a necklace. In creating a polysulfonate "necklace" with SuFEx, the researchers identified ethenesulfonyl fluoride-amine/aniline and bisphenol ether as good "beads" to use and found that using bifluoride salt as a catalyst made the previously slow reaction "click" into action. Researchers found that the high efficiency of the reaction results in a remarkable 99 percent conversion, from starting reactants to products, in less than an hour.
Researchers found that the new reaction requires 100 to 1,000 times less catalyst than other known methods, resulting in significantly less hazardous waste. Bifluoride salts are also much less corrosive than previously used catalysts, allowing for a wider range of starting substrate "beads," which researchers said they hope could lead to its adoption for a range of industrial processes.
"There are many new polymers that haven't been widely used by industry before," said Liu. "By reducing waste and improving product purity, we lower the cost and make this reaction much more industry friendly."
The Molecular Foundry is a DOE Office of Science User Facility that provides free access to state of the art equipment and multidisciplinary expertise in nanoscale science to visiting scientists from all over the world.
More information: Bing Gao et al, Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates, Nature Chemistry (2017). DOI: 10.1038/nchem.2796
Hua Wang et al. SuFEx-Based Polysulfonate Formation from Ethenesulfonyl Fluoride-Amine Adducts, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201701160
Journal information: Nature Chemistry , Angewandte Chemie , Angewandte Chemie International Edition
Provided by Lawrence Berkeley National Laboratory