Chemists discovered how the viper venom works
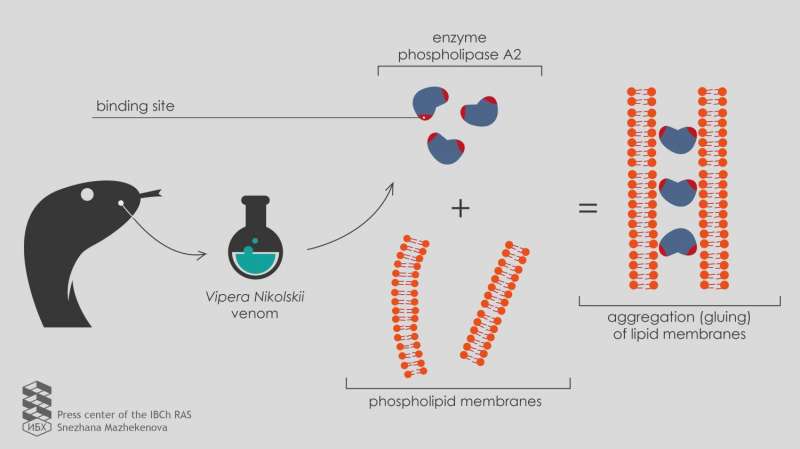
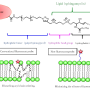
Russian scientists report that the enzyme phospholipase A2 from the Vipera nikolskii venom is able to adhere to lipid membranes and cause their aggregation, even if the activity of the enzyme is specifically blocked. This is due to the presence of two binding sites in the enzyme structure. Published in the journal Toxicon, the results help to understand how multicomponent snake venoms work.
Some animals avoid danger with help of camouflage, others with claws, teeth or hooves. And some insects, spiders, scorpions and snakes have poisons that can not only paralyze, but also kill the attacker. Many researchers are interested in the complex poison components, because they can be used to produce antidotes and create new drugs. Venoms contain neurotoxins – poisons that act on the nervous system and block the conduction of nerve impulses. These neurotoxins include certain types of enzyme phospholipase A2.
The researchers' interest in phospholipase A2 is due to the fact that it serves as a marker of inflammation. This means that the level of this enzyme in the blood rises with acute inflammation in the body. Therefore, several international scientific groups are trying to create systems for detecting the activity of phospholipase A2.
In 2016, researchers at the Laboratory of Lipid Chemistry of IBCh RAS have developed a fundamentally new fluorescence method for detecting the interaction of this enzyme with a double molecular layer of lipids (the so-called lipid bilayer) – the basic structure of the cell membrane. The method is based on energy transfer between two fluorescent dyes (fluorophores attached to the molecules of phospholipids) embedded in the lipid bilayer.
"To test the new development on as many samples as possible, we turned to the Laboratory of Molecular Toxinology IBCh RAS," says Ivan Boldyrev, a senior researcher at the Laboratory of Lipid Chemistry. "The head of the laboratory, Yuri Utkin, has collected a set of phospholipases A2 from poisons of various organisms, including two heterodimeric phospholipases A2 from Vipera nikolskii venom. Each of these enzymes consists of two heterofunctional subunits, polypeptide chains folded in a specific way. However, the toxic effect mechanism of these heterodimers is not clear."
The research produced unexpected results. Under the action of these "double proteins" (heterodimers), fluorescence did not occur, as with all other phospholipases, but instead decayed.
A more in-depth study using electron microscopy showed that heterodimeric phospholipases A2 from the Vipera nikolskii venom caused the aggregation of lipid membranes, meaning that they glue them together. However, this effect emerged only with negatively charged membranes.
"Uncharged membranes with no electric charge on the surface do not combine under the action of heterodimers," explains Anna Alekseeva, a junior researcher at the Laboratory of Lipid Chemistry.
"We managed to establish the specificity of heterodimeric phospholipases A2 for negatively charged membranes and determined pH conditions of the medium at which the enzyme manifests itself," says DariaTretyakova, a Ph.D. student at the Laboratory of Lipid Chemistry.
The new data will help to understand the effect of multicomponent snake venoms, and the developed methodology will be in demand for studying other lipid-protein interactions.
More information: A.S. Alekseeva et al. HeterodimericV. nikolskiiphospholipases A2 induce aggregation of the lipid bilayer, Toxicon (2017). DOI: 10.1016/j.toxicon.2017.05.015