Alloy compositions may influence particles' electronic states
A research team consisting of NIMS, Kyoto University and Oita University groups for the first time successfully measured the electronic states of alloy nanoparticles consisting of rhodium (Rh) and copper (Cu) that exhibit similar catalytic activities at different Rh-to-Cu ratios. The nanoparticles serve as a catalyst for exhaust gas purification.
A research team has for the first time successfully measured the electronic states of Rh-Cu alloy nanoparticles (NPs) that exhibit similar catalytic activities at different Rh-to-Cu ratios (by number of atoms). The nanoparticles serve as a catalyst for exhaust gas purification. The results indicated that it is difficult to correlate NPs' electronic states with their catalytic activities. More detailed analysis on the relationship between these two variables may lead to the discovery of new methods of rendering alloy NPs as effective catalytically as pure Rh NPs. These methods may not be based on the matching of alloy NP electronic states with those of pure Rh NPs.
The rare element Rh is a promising catalyst for the purification of exhaust gases from automobiles and other sources. However, because Rh is a highly valuable resource, its use needs to be minimized. Kitagawa's group at Kyoto University previously succeeded in synthesizing Rh-Cu alloy NPs, which is impossible to achieve using bulk materials. Nagaoka's group at Oita University confirmed that these alloy NPs are capable of serving as a catalyst for exhaust gas purification by oxidizing exhaust gas components such as CO and NOx. Their catalytic capability was comparable to that of pure Rh NPs, and did not diminish with decreasing Rh content. It had been in general assumed that changing the composition of alloy NPs would also change their electronic states, and that their catalytic activities were closely related to their electronic states. Based on these assumptions, many materials scientists had been interested in studying the electronic states of Rh-Cu alloy NPs. Technical issues made such a study difficult to implement in reality. Recently, Sakata's group at NIMS for the first time measured the electronic states of Rh-Cu alloy NPs at different Rh-to-Cu ratios.
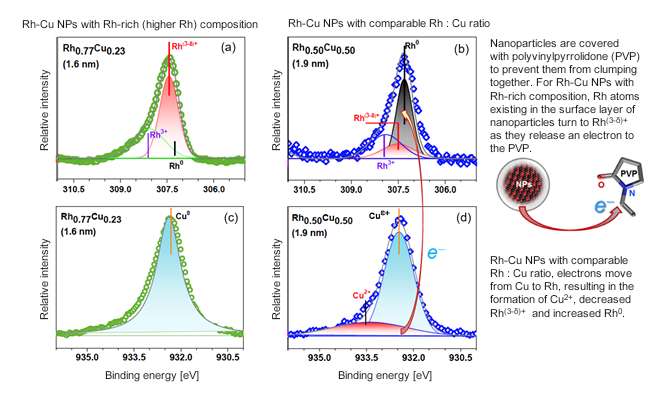
It is very difficult to accurately evaluate the electronic states of NPs using photoelectron spectroscopy employing low energy (soft) X-rays. This is because NP surfaces are coated with a protective material to prevent them from clumping together. To overcome this issue, we took photoelectron spectroscopy measurements of the NPs at NIMS's beamline located in the world's largest synchrotron radiation facility (SPring-8). The facility enabled us to collect electronic state data from the entirety of the NPs using high energy (hard) X-rays capable of penetrating the protective outer layer material. We examined the electronic states (oxidation states) of two types of Rh-Cu alloy NPs: NPs with a higher (about 80%) Rh and comparable Rh : Cu (about 50%) content. Similar oxidation states were found in NPs with the higher Rh content and in pure Rh NPs. On the other hand, NPs with the comparable Rh : Cu ratio had a lower proportion of Rh(3-δ)+ in an oxidation state and higher Rh0 than that of the NPs with the higher Rh content and had a higher proportion of Cu2+ in an oxidation state.
These results indicate that more detailed evaluations of electron states are vital to the creation of new catalytic and other functional materials. In the future, we plan to carry out a theoretical study on the relationship between NPs' catalytic activities and their electronic states. In addition, to accelerate the creation of new functional materials, we will promote the development of materials informatics by providing our data on the electronic structures and atomic arrangements of alloy NPs and various other materials.
This research was supported by the MEXT's Nanotechnology Platform Japan program, and JST's ACCEL project entitled "Creation of innovative functions of intelligent materials on the basis of element strategy" (Professor Hiroshi Kitagawa, research team leader).
This study was published in Scientific Reports on January 25, 2017.
Journal information: Scientific Reports
Provided by National Institute for Materials Science