3-D structure of enzyme opens path to new drug design in brain disease
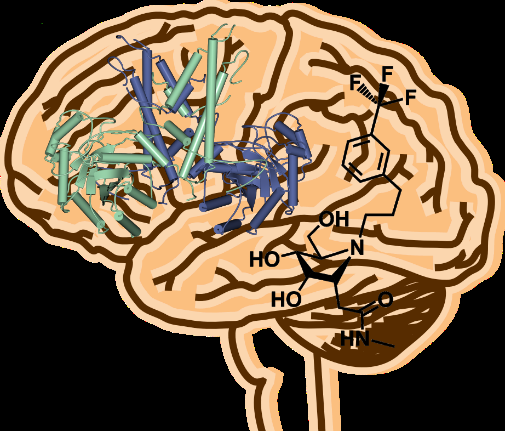
Researchers at the University of York and Simon Fraser University, Canada, revealed the 3-D structure of an enzyme that could provide a crucial step forward in treating neurodegenerative diseases.
Previous work from these research teams investigated a class of diseases called tauopathies, which occur when tau proteins spontaneously group together in the brain. It is often associated with Alzheimer's and other neurodegenerative diseases.
Research has shown that the tau protein can be modified by a sugar, natural to the body, called O-GlcNAc. This sugar can stabilise the protein to block it from clumping together and may thereby prevent disease. The human enzyme O-GlcNAc-hydrolase, however, is responsible for the removal of this crucial sugar from the protein, making it a prime target in preventing the progression of tau-related dementias.
In order to understand how this clumping of tau could be prevented or reduced by increasing O-GlcNAc, scientists at York investigated the structure of the human enzyme to reveal how it is organised to function in this way.
New breakthroughs
Professor Gideon Davies, from the University's Department of Chemistry, said: "Inhibiting the O-GlcNAc-hydrolase enzyme allows scientists to stabilise tau. We have solved the three-dimensional structure of the enzyme in order to aid structure-based drug design. The unusual and complex organisation should help us in the search for new drugs to treat neurodegenerative diseases.
"Drugs can be designed based on the 3-D structure of this human enzyme, which will ultimately pave the way for new breakthroughs in the treatment of diseases such as Alzheimer's."
Professor David Vocadlo, from Simon Fraser University, said: "In addition to serving as a blueprint for the development of antagonists, this long sought after structure reveals a surprising architecture that may lead to improved understanding of how this important enzyme is regulated in cells. Such insights could lead to more targeted therapeutics for various diseases."
Key to a lock
Dr Rosa Sancho, Head of Research at Alzheimer's Research UK who part-funded this work, said: "Drug discovery is a bit like designing a key to fit a lock, however, it is important to know the shape of the lock you are working with. This new study describes in detail the shape of O-GlcNAc-hydrolase and paves the way for the design of drugs that can fit this lock. Future studies will need to explore whether drugs that can inhibit this enzyme hold promise for treating Alzheimer's disease and other dementias, but this is an important step in the right direction.
"With 850,000 people in the UK currently living with dementia and no new treatments licensed in the last decade, there is an urgent need for new and innovative strategies to tackle the condition head on."
The research is published in Nature Chemical Biology.
More information: Christian Roth et al. Structural and functional insight into human O-GlcNAcase, Nature Chemical Biology (2017). DOI: 10.1038/nchembio.2358
Journal information: Nature Chemical Biology
Provided by University of York