February 17, 2017 report
Blue light allows for making carbon-nitrogen bonds without 'energetically unfavorable' reactions
(Phys.org)—A team of researchers at Princeton University and a Bristol-Myers Squibb associate have developed a means for creating reactive ammonium radical cations using flashes of blue light. In their paper published in the journal Science, the team describes their new technique and the ways they believe it could be used to create substituted amines. Travis Buchanan and Kami Hull with the University of Illinois offer a Perspectives piece on the work done by the team and the expected impact the new technique is likely to have on organic chemistry.
As Buchanan and Hull note, amines (which are molecules that have a C—N bond) are very important in the pharmaceutical industry—approximately 84 percent of pharmaceuticals contain amines. But, as they also note, conventional processes that are used to create carbon-nitrogen bonds involve what they describe as energetically unfavorable reactions—they are inefficient. The process typically involves hydroamination which is where a N—H bond is directly added to a C—C molecule, or even to a triple. In this new effort, the researchers report on a new method using a simple blue light from an LED that gets the job done in a more efficient way.
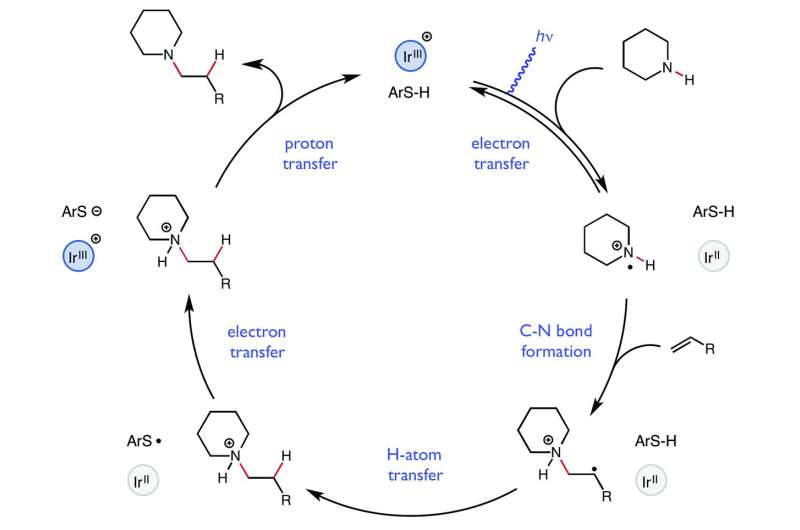
The team at Princeton used a photochemical approach that allowed for the creation of reactive ammonium radical cations using a flashing blue light (for 12 hours), which in turn was used to form the desired isomers. They used the blue light to excite an iridium complex to oxidize the amine, allowing for efficient bonding with the olefin. A thiophenol cocatalyst was then used to move the electron back. The researchers report that their technique could be uses for a variety of olefin and amine compounds allowing for using amines in pharmaceuticals in new and useful ways—some of which, they report, could not be created any other way. Furthermore, they note, the technique is completely atom economical—all of the atoms in the starting materials wound up in the end product.
Buchanan and Hull suggest the new approach could represent a transformative approach to amine synthesis, noting that the researchers used their technique to animate a sample of every existing olefin type.
More information: Andrew J. Musacchio et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines, Science (2017). DOI: 10.1126/science.aal3010
Abstract
The intermolecular hydroamination of unactivated alkenes with simple dialkyl amines remains an unsolved problem in organic synthesis. We report a catalytic protocol for efficient additions of cyclic and acyclic secondary alkyl amines to a wide range of alkyl olefins with complete anti-Markovnikov regioselectivity. In this process, carbon-nitrogen bond formation proceeds through a key aminium radical cation intermediate that is generated via electron transfer between an excited-state iridium photocatalyst and an amine substrate. These reactions are redox-neutral and completely atom-economical, exhibit broad functional group tolerance, and occur readily at room temperature under visible light irradiation. Certain tertiary amine products generated through this method are formally endergonic relative to their constituent olefin and amine starting materials and thus are not accessible via direct coupling with conventional ground-state catalysts.
Journal information: Science
© 2017 Phys.org