UV light for producing customized surfaces

Scientists of Karlsruhe Institute of Technology (KIT) have developed a new process to structure surfaces and to apply or detach functional molecules. They use UV light for the formation or breaking of so-called disulfide bridges, i.e. bonds of sulfur atoms. Both photodynamic reactions allow for a temporally and spatially controlled and reversible modification of the surface and, hence, can be used to produce functional interfaces. The process is reported in the journal Angewandte Chemie.
Interfaces of solids and their interactions with gases, liquids, or other solids are of relevance to many applications in e.g. electronics, biotechnology or biomedicine. These interactions can be controlled among others by a spatially resolved functionalization of surfaces. Photochemical, i.e. light-induced, reactions are applied for the temporal and spatial control of the surface modification. However, most of the photochemical reactions applied so far for surface functionalization cause irreversible modifications, as they produce covalent bonds that are very strong and hardly undergo chemical reactions.
Researchers of the Biofunctional Polymer Materials group headed by Dr. Pavel A. Levkin at the Institute of Toxicology and Genetics (ITG) and the Institute of Organic Chemistry (IOC) of KIT now present a new process in the renowned journal Angewandte Chemie. With the help of this process, temporally and spatially controlled and reversible patterning of surfaces is possible. "Reversible photochemical modifications can be used for many purposes," Pavel A. Levkin explains. "They are suited for the dynamic adjustment of interface properties, for activating and deactivating specific functions by certain stimuli, or for applying and detaching functional molecule groups."
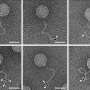
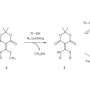
The scientists used UV light for the formation and breaking of disulfide bridges. These are compounds of two sulfur atoms that link two molecules. A disulfide bridge is formed by the oxidation of thiols, i.e. organic sulfur compounds which correspond to alcohols, but possess a sulfur atom instead of an oxygen atom. The disulfide bridge is detached again by means of reduction. UV-induced formation and reduction of disulfide bridges allow for the temporally and spatially controlled thiol-disulfide exchange. The researchers specifically applied disulfide to thiol-modified polymer surfaces and generated precise fluorescent patterns with small structures of up to 10 µm in size. Photopatterning also works in the opposite direction by applying thiols to disulfide-modified surfaces.
More information: Lei Li et al. UV-Induced Disulfide Formation and Reduction for Dynamic Photopatterning, Angewandte Chemie International Edition (2016). DOI: 10.1002/anie.201607276
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Karlsruhe Institute of Technology