January 31, 2017 report
New method for activating Earth-abundant metal catalysts
(Phys.org)—Many industrially relevant reactions require either a precious metal catalyst or an Earth-abundant metal catalyst in a low oxidation state. A catalyst with a Fe(0) complex, for example, is a good catalyst for hydroboration and hydrosilylation, but the methods required to reduce iron are prohibitive for practical use.
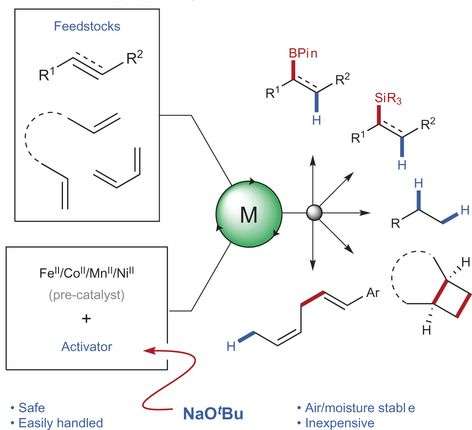
Researchers from the University of Edinburgh in the UK have demonstrated that sodium tert-butoxide serves as a robust and generally applicable activator for Earth-abundant metal catalysts. They tested pre-catalyst complexes with iron, cobalt, manganese, and nickel. Their method used commercially available reagents and mild conditions. They also demonstrated that their sodium tert-butoxide method for catalyst activation works with a variety of functional groups and metal ligands. Their work appears in Nature Chemistry.
If researchers want to use Earth-abundant metals, found in the first row of the transition metals, then they must use a pre-catalyst activator. First-row metals prefer to be in the most stable oxidation state. For iron and cobalt, Fe(II) and Co(II) are the most stable states. However, for catalytic activity, Fe(0) and Co(I) are preferred. This requires the use of either a strong reducing agent, such as sodium mercury amalgam, or an organometallic activator, such as lithium aluminum hydride or a Grignard reagent. Neither of these options is very practical for large-scale applications due to their sensitivity to air and water.
In looking for a better activator, Jamie H. Docherty, Jingying Peng, Andrew P. Dominey and Stephen P. Thomas sought a reagent that was chemically stable, easy to handle, scalable, and non-toxic. They eventually landed on sodium tert-butoxide based on their studies using a model reaction with iron-catalyzed hydroboration.
"An overarching goal of our research is to develop practical and simple synthetic methods for Earth-abundant metal catalysis," Dr. Stephen P. Thomas, principle investigator, told Phys.org. "With this in mind, we were keen to find an activation protocol which used air- and moisture stable reagents that are commercially available. Building upon our earlier work which used tertiary amines for this process, we found a variety of alkoxides were able to activate an iron(II) pre-catalyst (e.g., sodium methoxide, potassium tert-butoxide etc.), but the one that proved most robust was sodium tert-butoxide. From here, we demonstrated the applicability of this method toward a range of different catalysts, across four metals and for five synthetic transformations."
The "pre-catalyst" reagent is the metal in its stable oxidation state and its ligands. The ligand structure is often specific to certain reaction types. The Thomas group tested several pre-catalysts with their sodium tert-butoxide procedure using previously published hydroboration addition reactions. They used 1-octene as well as 4-phenyl-1-butene and myrcene as starting materials. Notably, the Thomas group's method involves a one-pot synthesis at 25oC for one hour.
They demonstrated comparable yields using their techniques to previously reported activators. Furthermore, they tested several cobalt pre-catalysts to see if they would work for hydroboration reactions and found that their sodium tert-butoxide method worked to activate cobalt-based pre-catalysts.
After optimizing their reaction, they then turned to hydrosilylation. Alkene hydrosilylation is one of the largest industrial processes currently in operation and typically uses a platinum catalyst in the industrial setting. As with hydroboration reactions, using an Earth-abundant metal for hydrosilylation required activation.
The Thomas group tested whether their method worked with hydrosilylation reaction using 1-octene or myrcene and several iron pre-catalysts. They found that sodium tert-butoxide again worked well as a catalytic activator and resulted in yields comparable to other methods.
From here, they then tested several cobalt-based pre-catalysts whose manifold is difficult to synthesize. They found that their one-pot reaction worked well for TerpyCoCl2, which is a novel cobalt catalyst for hydrosilylation. They then tested other first row transition metals and successfully completed an alkene hydrosilylation with an activated manganese catalyst and a nickel catalyst. This is the first time that a manganese catalyst is reported for alkene hydrosilylation and demonstrates that this method could be used to discover new types of catalysts.
Mechanistically, the tert-butoxide anion activates the boron or the silicon reagent to form either boron "ate" complexes or silicon "ate" complexes. This, in turn, serves to activate the metal pre-catalyst. Since these "ate" complexes are key to activating the metal catalyst, the Thomas group then decided to try using these complexes with reactions that do not involve borane or silane starting materials. They successfully completed a hydrovinylation reaction, an alkene hydrogenation, and a [2π+2π] cycloaddition reaction.
Overall, this reaction mechanism is a straight-forward process that utilizes a commercially available base to activate catalysts made of Earth-abundant metals. This has several implications for industrial chemistry, including new processes that do not use precious metal catalysts.
When asked about future research in this area, Dr. Thomas replied, "I think this activation method has made the barrier to discovery much lower. Our group is using this method to test new catalysts and to find new catalytic activity alongside developing a number of catalytic synthetic transformations. What's most encouraging is that other research groups are already starting to use this method."
More information: Jamie H. Docherty et al. Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide, Nature Chemistry (2017). DOI: 10.1038/nchem.2697
Abstract
First-row, earth-abundant metals offer an inexpensive and sustainable alternative to precious-metal catalysts. As such, iron and cobalt catalysts have garnered interest as replacements for alkene and alkyne hydrofunctionalization reactions. However, these have required the use of air- and moisture-sensitive catalysts and reagents, limiting both adoption by the non-expert as well as applicability, particularly in industrial settings. Here, we report a simple method for the use of earth-abundant metal catalysts by general activation with sodium tert-butoxide. Using only robust air- and moisture-stable reagents and pre-catalysts, both known and, significantly, novel catalytic activities have been successfully achieved, covering hydrosilylation, hydroboration, hydrovinylation, hydrogenation and [2π+2π] alkene cycloaddition. This activation method allows for the easy use of earth-abundant metals, including iron, cobalt, nickel and manganese, and represents a generic platform for the discovery and application of non-precious metal catalysis.
Journal information: Nature Chemistry
© 2017 Phys.org