Scientists unveil hidden step in enzyme mechanism
An international research team led by the University of Leicester has made a breakthrough advance by trapping an intermediate in the mechanism of enzymes called heme peroxidases and determining its structure using a beam of neutrons from the heart of a nuclear reactor.
The advance is announced today in an online publication in Nature Communications. The Leicester team members say they are delighted by their finding which could change the way we understand how these enzymes work through 'wonderful collaborations' with scientists at European facilities such as the Institut Laue-Langevin (ILL) in Grenoble and FRM-II in Munich, as well as the Diamond Light Source in Oxfordshire and the EPR centre at Manchester University. Professors Emma Raven and Peter Moody from the University of Leicester's Institute of Structural and Chemical Biology led the team that developed a new method to trap and analyse the enzyme's reaction steps.
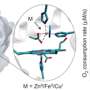
Professor Moody of the Department of Molecular and Cell Biology, said: "Using beams of neutrons instead of X-rays lets us see the position of hydrogen atoms without altering the chemical state. These enzymes go through two intermediate steps, a couple of years ago we used neutron cryo-crystallography to show the hydrogens in the first step (published in Science), and since then a great deal of work by our team has allowed us find a way to trap the next step. It had been believed that this second step did not hold hydrogen at the reactive centre, however this work clearly shows the hydrogen and so we have to re-think the way the enzyme works."
Heme enzymes have an iron atom in a special chemical group called a porphyrin, this is the same as in hemoglobin, the molecule that carries oxygen in our blood, but in heme peroxidase enzymes it is used to pull apart peroxide for many different biochemical processes, these include getting rid of damaging compounds in the cell and the making of new molecules that the cell needs.
Professor Raven from the University's Department of Chemistry said: "The exact nature of these enzyme intermediates has been the subject of a long-standing controversy and conflicting interpretation of indirect evidence. At least we have been able to see these directly, this really is the 'holy grail' of heme enzyme research."
More information: Nature Communications, DOI: 10.1038/ncomms13445
Journal information: Nature Communications , Science
Provided by University of Leicester