Study uncovers how cells organize the growth of their structural filaments
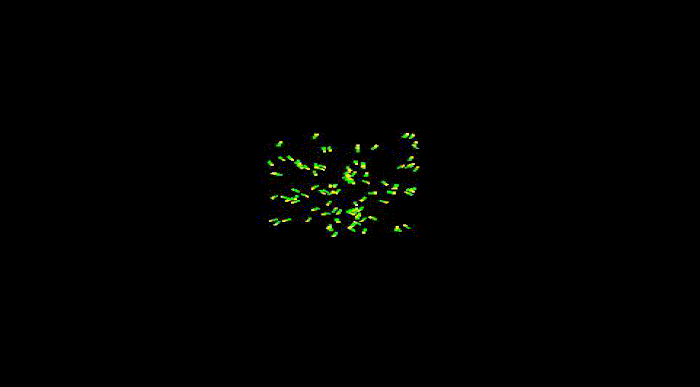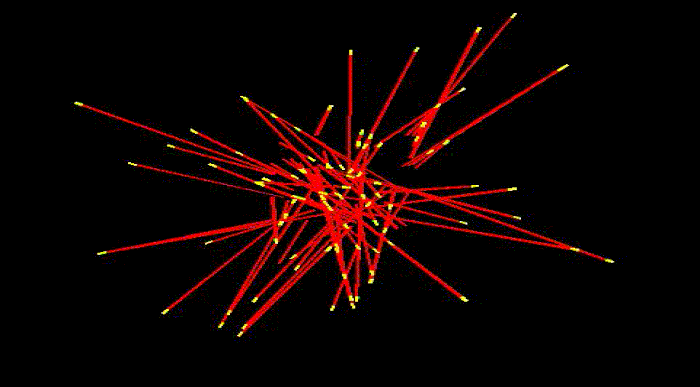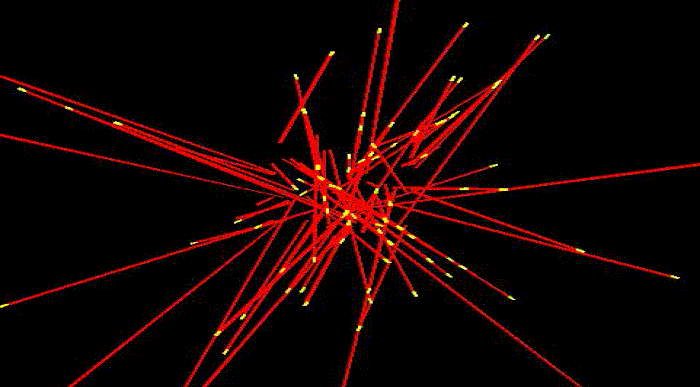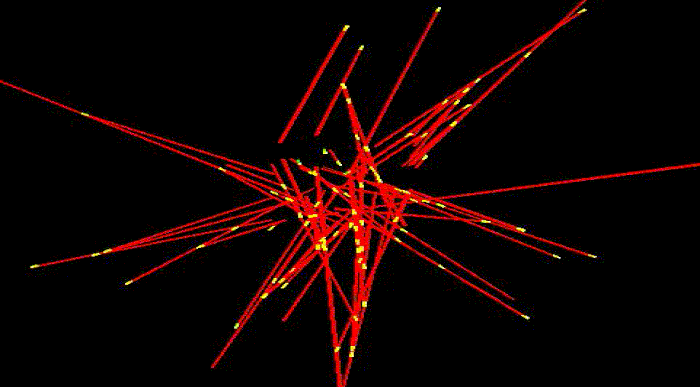
To take shape, to move and to reproduce, cells need internal scaffolding composed of slender filaments known as microtubules. Before the cell can use microtubules for these and other essential functions, it must first organize them into carefully crafted bundles, which become the basis for three dimensional shapes.
New work led by a Rockefeller University scientist offers insight as to how cells align their microtubules in order to bundle them. The study, published on October 6 in Cell, describes how two proteins work together to guide the growth of a new microtubule along an existing one.
Microtubules are highly dynamic structures that rapidly assemble and disassemble. Each is composed of building blocks bound together, like a string of small magnetic beads—and just as magnets have poles, these building blocks have "plus" and "minus" ends. Growth occurs at the plus end, where new blocks are added. As this happens, new microtubules radiate out from a common center, extending in all directions as they lengthen.
Somehow, the cell wrangles the growing microtubules, bringing them into clustered bundles that extend in common directions. Thus far, it's been unclear how this process occurs.
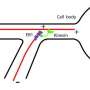
A team of researchers led by Alipasha Vaziri, an associate professor and head of the Rockefeller's Laboratory of Neurotechnology and Biophysics, has found that a molecular motor, called Kinesin-14, helps to guide the formation of a new microtubule along an existing one, and so directs the formation of bundles.
This protein's unusual ability to walk in both directions along the filament makes this possible. "It has a preference for movement towards the minus end, but the smallest bit of force can make it change direction and move toward the plus end," says Vaziri says, who was working at the Research Institute for Molecular Pathology in Vienna at the time the research was conducted.
His team found that, from its vantage point at the minus-end of an existing microtubule, Kinesin-14 attaches to a second protein that is bound to the plus end of a growing microtubule. This interaction nudges Kinesin-14 backward, prompting it to guide the growth of the new microtubule in parallel with the older one.
The team showed that a wide range of animal cells employ this mechanism. "While we made our original observation in yeast, we were able to show the same phenomenon for human and fly cells. This means that this is a general mechanism conserved throughout evolution," Vaziri says.
More information: Maxim I. Molodtsov et al. A Force-Induced Directional Switch of a Molecular Motor Enables Parallel Microtubule Bundle Formation, Cell (2016). DOI: 10.1016/j.cell.2016.09.029
Journal information: Cell
Provided by Rockefeller University