Research reveals unprecedented observations of how a hot molecule cools in a liquid
The most detailed exploration to date of how energy flows from a hot molecule into a surrounding liquid has been undertaken by a team of scientists at the University of Bristol.
Led by Professors Mike Ashfold and Andrew Orr-Ewing from the School of Chemistry, the research, published recently in Nature Chemistry, has significant implications for a fundamental understanding of the mechanisms of cooling and provides fresh insights into the extraordinarily complex behaviour of liquids.
If a hot object is dropped into a liquid such as water, it quickly cools and the liquid warms up until both have the same temperature.
This equilibration of temperatures occurs because the hot object loses energy to the surrounding liquid.
The energy is transferred by collisions between the molecules of the liquid and the submerged object, but is difficult to study because these collisions happen very quickly (typically in less than a trillionth of a second).
Using very short laser pulses, the Bristol team has been able to watch how energy flows from a hot object into a liquid in unprecedented detail.
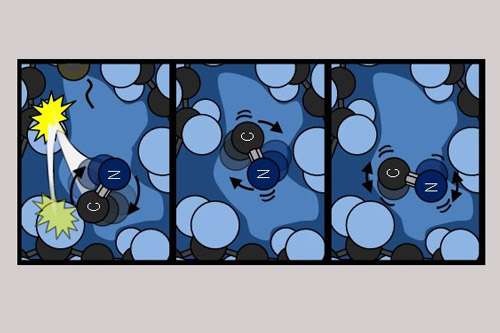
Professor Andrew Orr-Ewing said: "In our experiments, small dissolved molecules were given a very large amount of energy using a short burst of ultraviolet light.
"The energized molecules initially spin very fast and move with high speeds, but rapidly encounter molecules of the surrounding solvent.
"They ricochet off the solvent molecules and transfer energy in the process, so that they spin more and more slowly until they run out of excess energy. The process is much like a spinning top slowing down as it bounces off obstacles such as walls or furniture, but is over in much less than a billionth of a second."
The experimental measurements were reproduced using simulations run on a computer, which helped the Bristol team to understand the forces acting between the hot solute molecules and the surrounding solvent molecules.
More information: Michael P. Grubb et al. Translational, rotational and vibrational relaxation dynamics of a solute molecule in a non-interacting solvent, Nature Chemistry (2016). DOI: 10.1038/NCHEM.2570
Further details of the research in the Bristol group can be found at www.bristoldynamics.com
Journal information: Nature Chemistry
Provided by University of Bristol