Chemical engineers explore market for pure levoglucosan

Chemical engineers and chemists from Rice University and China's Dalian Institute of Chemical Physics have made something so useful and unusual they aren't yet sure how much it's worth.
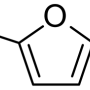
In a new paper in the journal Green Chemistry, a team led by Rice's Michael Wong describes a new process for making extremely pure levoglucosan (LGA), a naturally occurring organic compound that has been so rare and expensive that drugmakers and chemical engineers typically haven't considered using it.
"A couple of years ago, we got to thinking about chemistries that could turn biomass into something of greater value than heat or biofuels," said Wong, professor and chair of Rice's Department of Chemical and Biomolecular Engineering and professor of chemistry. "Most chemicals are made from oil and gas, but you can't make LGA from petrochemicals. LGA has a very interesting structure that makes it a much better starter molecule than sugar, but it's been hard for researchers to work with LGA when quantities are limited. LGA is so difficult and inefficient to make that whatever small amounts were commercially available were very expensive."
Wong said LGA's value derives from the options it presents to drugmakers and chemical engineers who specialize in chemical synthesis, a branch of chemistry that lies at the center of some of the world's largest industries, including pharmaceuticals, petrochemicals, plastics and polymers. The complex chemicals these industries produce are built up from smaller chemicals, much like a Lego model is built from individual bricks. LGA is an organic precursor chemical, one of the organic "bricks" that a chemist could use in a synthesis reaction.
Wong said LGA is particularly handy because it contains a number of chiral carbons, which means the carbons come in left-handed and right-handed forms that are not mirror images of one another. Just as specially shaped Lego bricks are rare but useful to build unique structures, LGA molecules could open new avenues to synthetic chemists, he said. Glucose also contains chiral carbons, but it is less stable to do chemistry with.
Wong and co-lead scientist Conrad Zhang of China's Dalian Institute of Chemical Physics worked with graduate student Li Chen to design a series of experiments to see if an efficient method could be found for making pure LGA in bulk.

"We've shown that we can make LGA that is at least 95 percent pure, and possibly much more pure, from three different feedstocks—cellulose, glucose and starch," Wong said. "This comes from our much better understanding of sugar chemistry at high temperatures, which we summarize in our paper as the ring-locking concept. We like to think we solved the purity problem, and now we're focusing on the quantity problem."
Wong said the group's process should be far more economical and operationally simpler than the current small-scale route for making LGA. Producers today begin with starch and apply intense heat under oxygen-free conditions. Heating requires lots of energy, and the result is a chemical soup that contains a few percent LGA. That soup must further be treated to separate the waste and yield highly pure LGA.
Chen, lead author of the new study, said, "Our approach is fundamentally different. We use a few chemical tricks to prepare our feedstocks in order to produce extremely pure LGA. So we've gone from 10 steps to two steps, which drastically cuts down the cost."
In a 2004 Department of Energy analysis, LGA was among the 30 most valuable chemicals identified as possible products of biorefineries, "green" chemical facilities that could compete with chemical plants that make products from nonrenewable oil and gas. Wong said LGA's unique properties could be useful for making "green" plastics (plastics made solely from biomass) if quantity were no longer an issue. He said some drugmakers have looked at using LGA as a building block for making at least a dozen drugs, including some of the widely prescribed type 2 diabetes drugs known as SGLT2 inhibitors.
The work was initiated with a National Science Foundation grant, which recently led to Wong and Chen receiving an NSF Innovation Corps grant to explore potential markets for LGA and other anhydrosugar compounds. I-Corps grants let academic researchers explore the commercial possibilities of a promising discovery. Each I-Corps team goes through an immersive seven-week process that includes online training with business mentors and interviews with at least 100 potential customers.
"The experience has been invaluable," Chen said. "It's intense, but instructive."
Wong said he and Chen may form a startup company if the I-Corps process reveals a significant market.
More information: Li Chen et al, Ring-locking enables selective anhydrosugar synthesis from carbohydrate pyrolysis, Green Chem. (2016). DOI: 10.1039/C6GC01600F
Journal information: Green Chemistry
Provided by Rice University