August 11, 2016 report
Cavitands enhance selectivity and hydrolysis rate of one ester on a long-chain diester
A challenge for chemists is trying to selectivity react one of two identical functional groups on the same molecule. For example, a diester with a long carbon chain in between the two ester groups has two chemically equivalent sites. Hydrolysis of this diester results in a mixture of products where one or both of the esters are converted to carboxylic acids. This results in lower overall yields.
New research by Qixun Shi, Matthew P. Mower, Donna G. Blackmond, and Julius Rebek, Jr. of Fudan University and Scripps Research Institute describe the use of cavitands as a way to overcome the monofunctionalization problem. They found that cavitands force a long-chain α,ω-dimethyl ester to fold onto itself changing the molecule's behavior compared to bulk solution. The cavitand results in greater selectivity and reaction rates for both the acid and base hydrolysis of diesters. Their work appears in the Proceedings of the National Academy of Sciences.
While there are ways to functionalize one of two identical functional groups on a molecule, these methods rely on there being some difference between the two functional groups. But, in the case of multi-carbon diesters, these procedures do not work because the functional groups are so far apart from each other that they essentially behave as two reactants (i.e., they have equivalent rate constants). One possible solution is to entrap the long-chain reactant within a molecular container causing the reactant to fold in a particular fashion. This is similar to how the body entraps a peptide chain in a chaperone protein during protein synthesis or how enzymes will catalyze an otherwise difficult reaction by causing a guest molecule to fold into a conformation that buries some of the reactive sites.
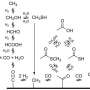
Cavitands are macrocyclic molecules shaped like a cup. The lip of the cup has functional groups that serve to broaden the rim and prevent dimerization. The chemical composition within the cavity as well as the cavity's shape promotes guest folding into a particular conformation. In the current research, the authors found that the α,ω-dimethyl ester guest folded into a J-shape, with one end outside of the cup while the other end remains within the cup. This allowed for both acid promoted and base-promoted (i.e., saponification) hydrolysis of only one end of the ester.
Using NMR to follow the reaction process, Shi, et al. first tested acid hydrolysis using DCl added to D2O in a solution containing diester and the cavitand. One end of the ester is deep within the cavitand while the other is accessible for hydrolysis. Once one end is hydrolyzed the other ester is rendered less reactive; however, because the J-shape conformation is dynamic, some of the other end of the diester does hydrolyze. The yield for the monoester product is still significantly greater than in bulk solution based on rate data.
Kinetic studies using NMR peak integration of the acid hydrolysis reaction indicate that the ester portion of the molecule remains suppressed within the cavitand three-fourths of the time. The reaction rate is more than a factor of ten greater than the comparable reaction in bulk solution. The authors suggest that this may be due to better solvation of the exposed ester. With the organic molecule-cavitand complex, the reaction can be carried out in water. Without the cavitand, the acid hydrolysis reaction requires the addition of an organic co-solvent.
The saponification reaction demonstrated even better selectivity and enhanced reaction rates compared to the acid hydrolysis reaction. The major product was monester with <5% of the product as diester or diacid. NMR analysis showed this exceptional selectivity has to do with phase transfer. In both the acid hydrolysis and saponification reactions, the cavitand allows the organic diester to react in an aqueous solvent. However, the difference between the two is that in the saponification reaction a monoanion is formed (RCOO-). The monoanion/cavitand complex then precipitates out of solution resulting in greater yields.
From this research, the authors have demonstrated that confined molecules behave differently than in bulk solution and this can be exploited for synthetic conundrums such as monofunctionalization of seemingly equivalent functional groups. The conformation of the guest molecule is guided predominantly by the shape of the cavitand, although chemical properties, such as hydrophobicity, do play a role. While the cavitand is difficult to modify, Dr. Julius Rebek points out that the cavitand used in this study is useful for other monofunctionalization reactions.
More information: Qixun Shi et al, Water-soluble cavitands promote hydrolyses of long-chain diesters, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1610006113
Journal information: Proceedings of the National Academy of Sciences
© 2016 Phys.org